Immunological Exploration: Solutions for Inducing Macrophage Differentiation
Macrophages, as central components of the immune system, play critical roles in immune defense and the regulation of inflammation. To gain a deeper understanding of their biological properties and functions, the cultivation of murine macrophages has become an indispensable research tool. Through carefully designed culture experiments, researchers can simulate various immune environments to study macrophage proliferation, differentiation, and immune responses in detail.
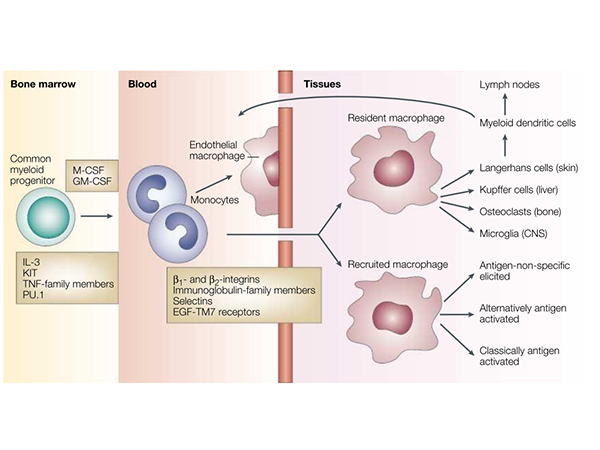
Simplified Life History of Macrophages
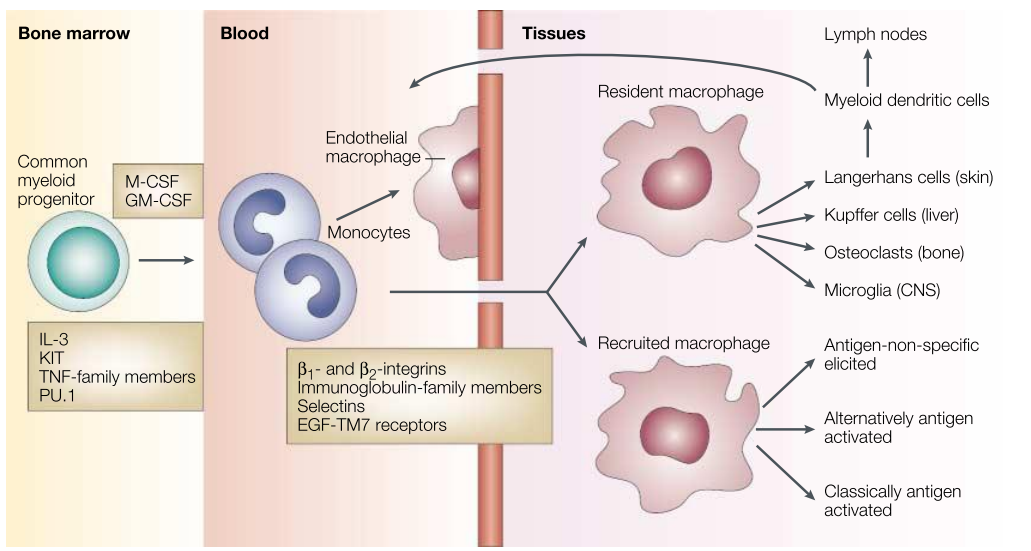
The differentiation, distribution, and activation of macrophages are key aspects of their biology. Macrophage growth and differentiation depend on lineage-determining cytokines, such as Macrophage Colony-Stimulating Factor (M-CSF) and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), as well as interactions with the stromal components of hematopoietic organs. IL-3, KIT, Tumor Necrosis Factor (TNF) family proteins, TNF receptor-associated molecules, and key transcription factors such as PU.1 and other ETS family members contribute to macrophage formation. A common progenitor gives rise to tissue macrophages, myeloid dendritic cells (DCs), and osteoclasts, which are distinct, irreversibly differentiated sublineages. Once distributed via the bloodstream, monocytes continuously enter all tissue compartments of the body. Resident macrophage populations in different organs—such as Kupffer cells (liver), alveolar macrophages (lungs), and microglia (central nervous system, CNS)—adapt to their local microenvironments.
Innate and Adaptive Immune Activation of Macrophages
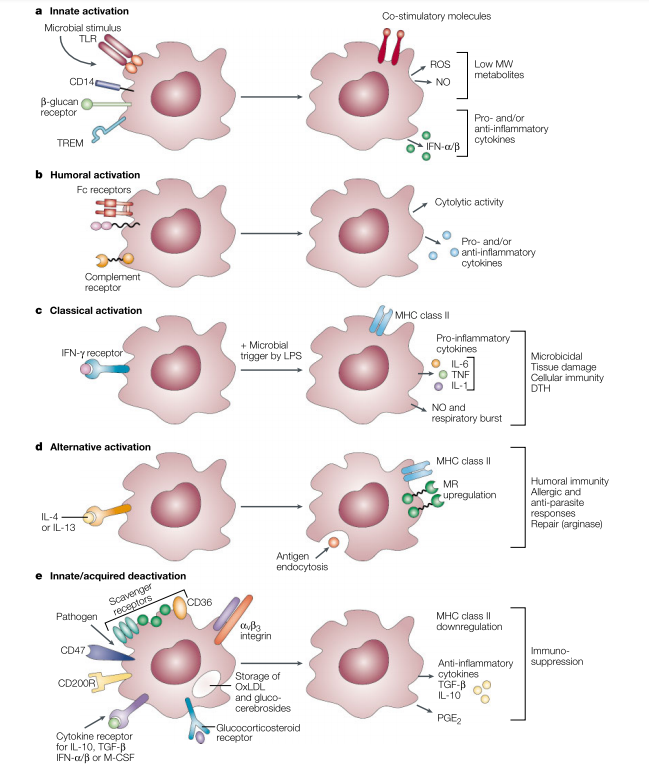
Microbial stimuli are recognized by pattern recognition receptors, such as Toll-like receptors (TLRs), CD14/lipopolysaccharide (LPS) binding protein, and a range of non-opsonic receptors. These stimuli induce the production of pro-inflammatory cytokines, such as IFN-α/β, reactive oxygen species (ROS), and nitric oxide (NO), followed by a regulated anti-inflammatory response. Enhanced expression of co-stimulatory surface molecules facilitates antigen presentation. Scavenger Receptor A (SR-A) and Mannose Receptor (MR) promote the phagocytosis and endocytosis of both host and exogenous ligands. Humoral activation and phagocytosis are mediated by certain Fc and complement receptors, while others downregulate the response. Classical activation is mediated by priming stimuli such as IFN-γ, followed by microbial triggers like LPS. Alternatively, activation is mediated by IL-4 and IL-13, acting through a common receptor chain (IL-4Rα). Deactivation can be innate or acquired. The uptake of apoptotic cells or lysosomal storage of host molecules induces anti-inflammatory responses. Macrophages interact with T cells, fibroblasts, and stroma through a series of receptors to modulate cellular activity. Cytokines and glucocorticoids are potent regulators of activation. Pathogens can deactivate macrophages through various mechanisms.
Experimental Protocol
Detailed Steps for Inducing Differentiation of Mouse Bone Marrow-Derived Macrophages for Flow Cytometry
Seed isolated bone marrow cells at a concentration of 2×10^6/ml in IMDM medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, along with 10 ng/ml M-CSF.
Replace the BMDM growth medium with fresh medium on day 3.
On day 7, assess the formation of mature BMDMs by staining with fluorochrome-conjugated antibodies and analyzing CD11b and F4/80 expression using flow cytometry.
On day 7, after confirming mature BMDM formation, replace the medium with fresh stimulation medium.
For M1 polarization, co-culture with 100 ng/ml LPS or 100 ng/ml LPS plus 50 ng/ml IFN-γ. For M2 polarization, co-culture with 10 ng/ml IL-4 and 10 ng/ml IL-13.
Detach stimulated cells from the culture dish using 0.05% trypsin, then wash the cells twice with PBS containing 10% fetal bovine serum.
Stain cells with fluorochrome-conjugated antibodies and analyze CD11b, F4/80, CD86, and CD206 expression using flow cytometry to assess polarization levels.
Note: The above steps are for reference only; reagent concentrations should be optimized based on experimental conditions.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA040056 | GM-CSF Protein, Mouse | Host : Mouse | $876 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040128 | M-CSF Protein, Mouse | Host : Mouse | $144 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040192 | IL-4 Protein, Mouse | Host : Mouse | $152 |
| Expression System : CHO | |||
| Conjugation : Unconjugated | |||
| UA040158 | IL-13 Protein, Mouse | Host : Mouse | $192 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040101 | GM-CSF Protein, Human | Host : Human | $156 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040002 | GM-CSF Protein, Human | Host : Human | $156 |
| Expression System : CHO | |||
| Conjugation : Unconjugated | |||
| UA040042 | M-CSF Protein, Human | Host : Human | $1,000 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040016 | M-CSF Protein, Human | Host : Human | $1,000 |
| Expression System : CHO | |||
| Conjugation : Unconjugated | |||
| UA040026 | IL-4 Protein, Human | Host : Human | $180 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040112 | IL-13 Protein, Cynomolgus | Host : Cynomolgus | $180 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated |




