The PD-L1 IHC antibody has been officially launched on the market!

Introduction to PD-1/PD-L1
The mechanism of action of PD-1/PD-L1 inhibitors involves blocking the interaction between PD-L1 expressed on tumor cells and PD-1 receptors on T cells. Tumor cells exhibit surface expression of PD-L1, which binds to PD-1 on T cells, delivering inhibitory signals that induce T cell apoptosis and suppress their activation and proliferation. By disrupting this interaction, PD-1/PD-L1 therapeutics restore T cell-mediated antitumor immunity, thereby achieving therapeutic objectives .
Currently approved PD-1/PD-L1 inhibitors are widely used in treating malignancies such as lung cancer, Hodgkin lymphoma, and esophageal carcinoma. Since the approval of China's first PD-1 inhibitor in June 2018, 15 PD-1/PD-L1 agents have been marketed domestically, including AstraZeneca's combination therapy targeting CTLA-4 and PD-L1. In 2022, Merck's pembrolizumab (Keytruda) achieved global sales of $20.937 billion, securing its position as the second highest-grossing drug in this class worldwide.
Key Considerations in PD-L1 Pathological Diagnosis
PD-L1 immunohistochemical (IHC) detection serves as a predictive biomarker for PD-1/PD-L1 therapy. The Expert Consensus on PD-L1 Immunohistochemical Testing in Solid Tumors (2021 Edition) outlines the following critical issues and consensus points:
High-specificity and high-sensitivity PD-L1 antibodies are pivotal for companion diagnostics. The consensus further clarifies thresholds for commercially available antibodies:
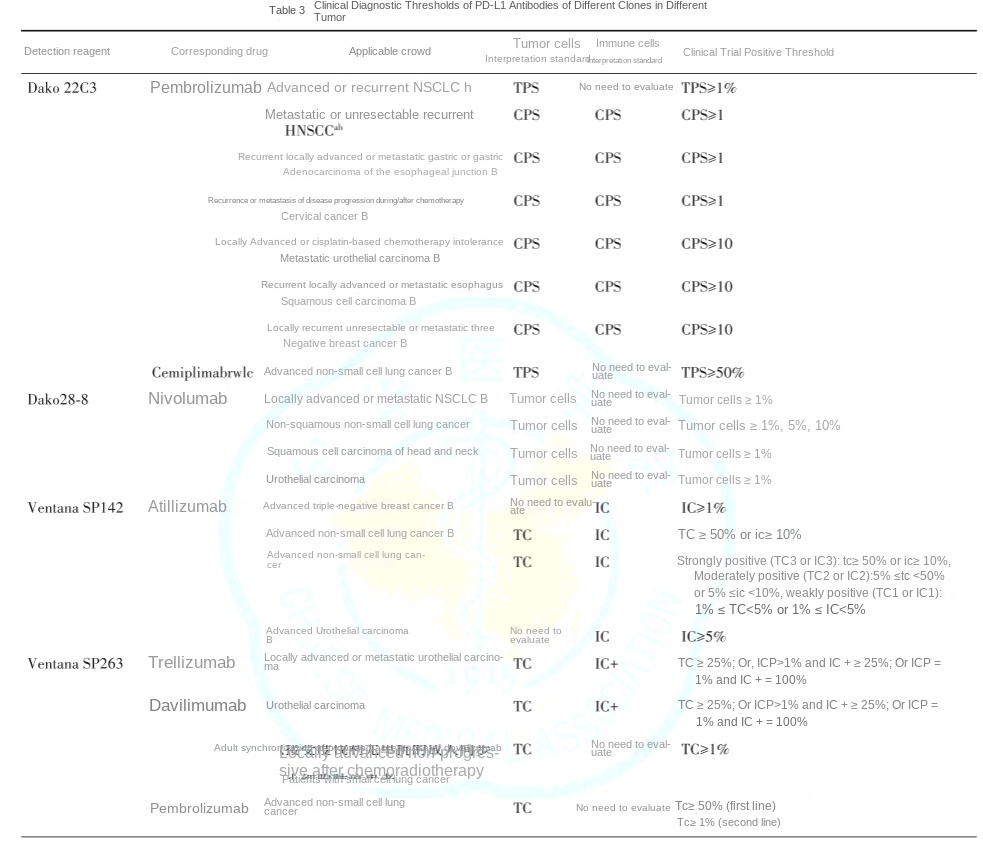
Note:
a. China's National Medical Products Administration (NMPA) has approved the Dako PD-L1 22C3 IHC assay as a companion diagnostic for pembrolizumab in first-line treatment of EGFR/ALK-negative locally advanced or metastatic non-small cell lung cancer (NSCLC) and esophageal squamous cell carcinoma.
b. The U.S. FDA has approved PD-L1 IHC assays as companion diagnostics for immune checkpoint inhibitors in corresponding tumor indications.
c. NMPA has approved the Ventana SP263 IHC assay as a companion diagnostic for tislelizumab in platinum-based chemotherapy-refractory locally advanced or metastatic urothelial carcinoma.
Scoring Systems:
PD-L1 expression levels are quantified using distinct scoring criteria across tumor types to correlate with therapeutic efficacy:
1、Tumor Proportion Score (TPS): Percentage of PD-L1-positive tumor cells.
2、Immune Cell Score (IC): PD-L1 expression in tumor-associated immune cells.
3、Combined Positive Score (CPS): Integrates PD-L1 expression in both tumor cells (TC) and immune cells (IC).
High-Sensitivity and Specific PD-L1 Antibodies
Starter's high-specificity PD-L1 antibody demonstrates exceptional sensitivity and specificity in human small cell lung cancer (SCLC) tissues, enabling precise identification of membranous PD-L1 protein expression on tumor cells with clear demarcation from cytoplasmic and other negative compartments. This antibody is suitable for developing in vitro companion diagnostic kits and commercial antibody production.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA010222 | PD-1 Fc Chimera Protein, Mouse | Host : Mouse | $500 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA010004 | PD-1 His Tag Protein, Human | Host : Human | $180 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA010002 | PD-1 Fc Chimera Protein, Human | Host : Human | $180 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| S0B2037 | PD-1 Recombinant Rabbit mAb (SDT-035-25) | Host : Rabbit | $880 |
| Conjugation : Unconjugated | |||
| S0B2068 | S-RMab® PD-L1 Recombinant Rabbit mAb (SDT-119-16) | Host : Rabbit | Inquiry |
| S0B2067 | S-RMab® PD-L1 Recombinant Rabbit mAb (SDT-119-38) | Host : Rabbit | $880 |




