Revitalizing Insights: New Discoveries in the Biology and Clinical Applications of IL-18
Introduction
Several recent discoveries have reignited interest in the pathogenic potential and possible clinical applications of IL-18. IL-18 is a member of the IL-1 family of cytokines, renowned for its potent ability to induce IFNγ production. However, both fundamental research and recent clinical observations suggest a more complex scenario. The unique biological characteristics of IL-18 at the levels of transcription, activation, secretion, neutralization, receptor distribution, and signaling contribute to explaining its pleiotropic roles in mucosal and systemic inflammation. Blood biomarker studies have revealed a cytokine whose significant elevation is associated with detectable "free IL-18," defining a group of autoinflammatory diseases where IL-18 dysregulation may be a primary driving feature, termed "IL-18opathies." This impressive specificity may accelerate diagnosis and identify patients suitable for therapeutic IL-18 blockade. Pathologically, human and animal studies have found that CD8+ T cells are preferentially activated over other IL-18-responsive lymphocytes. Therapeutic strategies utilizing IL-18 agonists that exploit production sites or subvert endogenous IL-18 inhibition show promise in enhancing cancer immune responses. Thus, the unique aspects of IL-18 biology are finally beginning to have clinical impacts in the precise diagnosis, disease monitoring, and targeted treatment of inflammatory and malignant diseases.
About IL-18
Between transcription and release, the processes of translation to secretion for IL-1 family members IL-1β and IL-18 are closely matched. Pro-IL-1β and Pro-IL-18 are inactive precursors that enter the cytoplasm. Both require proteolytic cleavage to become active cytokines and rely on membrane pores to exit their cells of origin. However, differences in pre-translational and post-release events have biological and clinical significance.
IL-18 is often considered a macrophage-derived cytokine, but current data indicate that IL-18 production varies significantly depending on the tissue and inflammatory signals received by macrophages. Moreover, nearly all barrier epithelia also contain substantial amounts of IL-18, sometimes acting as inducible effectors and other times as pre-formed alarms. The implications of this pleiotropy are only beginning to be elucidated.
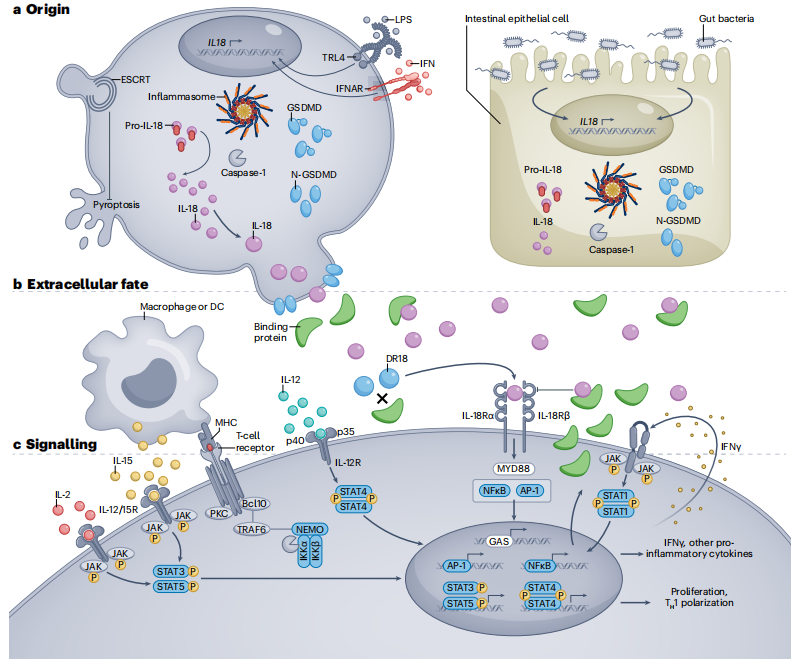
Figure 1: Origin, Extracellular Context, and Signaling of IL-18
a. Whether constitutively produced (epithelial cells and some tissue macrophages) or induced by complex stimuli (e.g., lipopolysaccharide (LPS), interferon (IFN), or intestinal dysbiosis), pro-IL-18 is typically cleaved by caspase-1 (or caspase-8, granzyme-B, proteinase-3, or chymase) into its active form, allowing it to exit the cell through gasdermin D (GSDMD) pores in an inflammasome-dependent manner. The ESCRT pathway can prevent pyroptosis caused by GSDMD pores.
b. Once released, IL-18 is usually bound and neutralized by IL-18 binding protein (IL-18BP), which is itself produced by most cell types in response to IFNγ. Non-bound or "decoy-resistant" IL-18 (DR-18) signals through the IL-18 receptor heterodimer (IL-18R), often acting synergistically with other signals.
c. IL-18R signals through the traditional IL-1 family/MyD88-dependent pathway. IL-18 can drive NF-κB and/or MAPK signaling, promoting proliferation, pro-inflammatory cytokine expression, and/or other effector programs (e.g., cytotoxicity). The diverse effects of IL-18 depend on the type and state of the cells it acts upon, as well as the presence of other signals such as TCR, IL-2, myeloid-derived IL-15, IL-12, and/or IFNγ (forming a positive feedback loop).
Applications of IL-18
IL-18 as a Biomarker
Clinicians routinely interpret levels of acute-phase reactants and inflammatory biomarkers. While most of these tests are valuable for monitoring disease activity, they lack specificity. Measurement of total IL-18 is rapidly becoming essential for diagnosing a subset of rheumatic and/or autoinflammatory diseases, particularly in pediatric rheumatology.
IL-18 and Disease
In many cases, profound and chronic elevations in total IL-18 (accompanied by detectable free IL-18) delineate a spectrum of primarily autoinflammatory diseases appropriately termed "IL-18opathies." Most of these diseases involve an increased susceptibility to macrophage activation syndrome (MAS), a secondary form of hemophagocytic lymphohistiocytosis (HLH) associated with rheumatic diseases. HLH and MAS are cytokine storm syndromes characterized by fever, hyperferritinemia (often >1,000 ng/ml), peripheral cytopenia, hepatosplenomegaly, hepatitis, disseminated intravascular coagulation, central nervous system involvement, and frequently, organ failure and death. Severe genetic defects in granule-mediated cytotoxicity are often described as the prototypical cause of HLH, although such defects account for only a small fraction of all HLH cases.

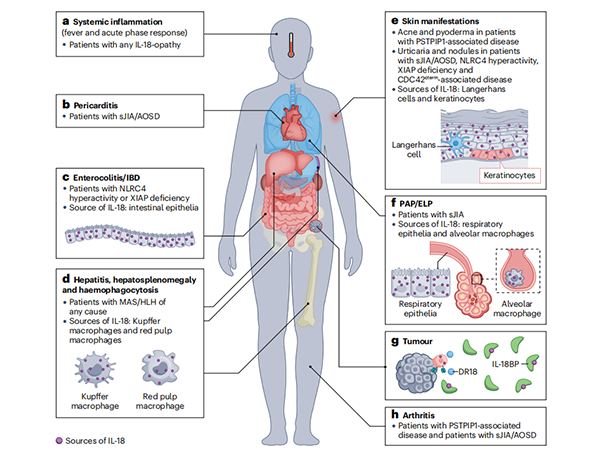
Figure 2: Pathological Manifestations of IL-18opathies Associated with IL-18 Sources
a. Although fever is less prominent in PSTPIP1-associated diseases, signs of systemic inflammation are present in all IL-18opathies.
b. Pericarditis occurs in some patients with systemic juvenile idiopathic arthritis/adult-onset Still's disease (sJIA/AOSD).
c. Colitis and inflammatory bowel disease (IBD) occur in patients with severe NLRC4 hyperactivity or XIAP deficiency. Colonic and small intestinal epithelial cells produce substantial amounts of pro-IL-18 under steady-state conditions.
d. Hepatitis, hepatosplenomegaly, and hemophagocytosis can occur in patients with macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH) of any cause. Hepatic Kupffer cells and splenic red pulp macrophages can produce significant amounts of pro-IL-18.
e. Cutaneous manifestations of IL-18opathies may include various transient, urticarial, or nodular rashes in sJIA/AOSD and monogenic MAS patients. PSTPIP1-associated diseases present with cystic acne and pyoderma gangrenosum, but MAS has not been reported in these patients. Keratinocytes and Langerhans cells produce substantial amounts of pro-IL-18 under steady-state conditions.
f. Pulmonary alveolar proteinosis (PAP) with endogenous lipoid pneumonia (ELP) has been observed in sJIA-associated lung disease. Both respiratory epithelial cells and alveolar macrophages produce significant amounts of IL-18 under steady-state conditions.
g. Tumors secrete large amounts of IL-18BP, which prevents IL-18 from promoting anti-tumor immunity but can be overcome by "decoy-resistant" IL-18 (DR-18) or IL-18-producing CAR-T cells.
h. Septic arthritis occurs in many patients with PSTPIP1-associated diseases. Although arthritis is a diagnostic criterion for sJIA/AOSD, its incidence is lower in sJIA/AOSD patients with higher IL-18 levels.
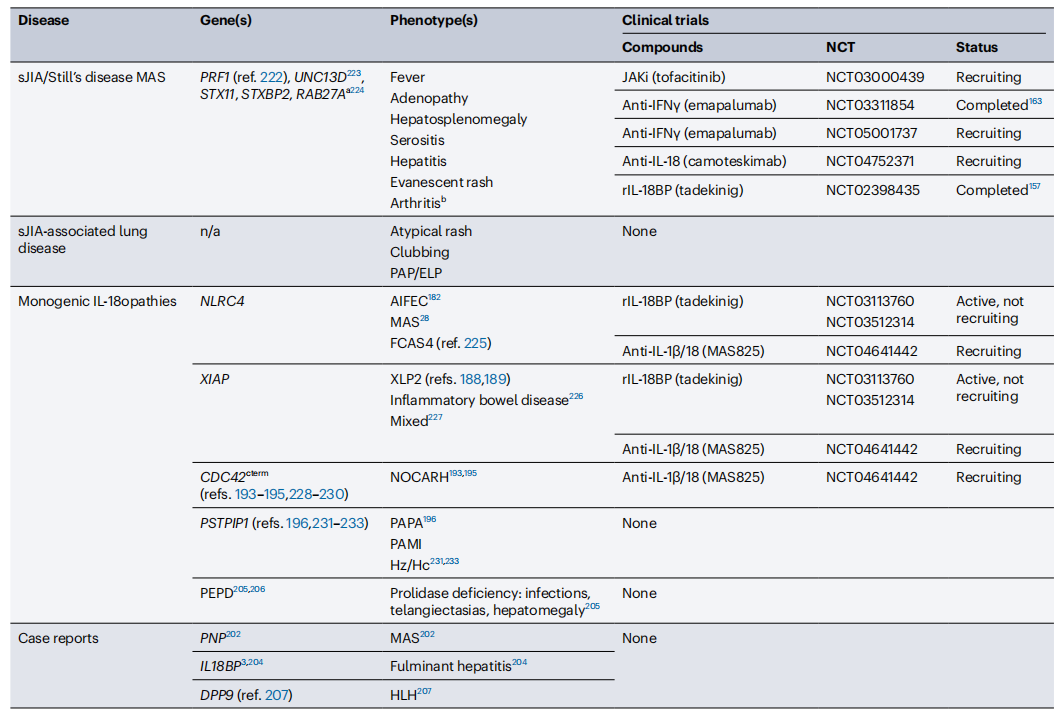
IL-18-Related Diseases and Ongoing Clinical Studies
(Currently, 461 clinical studies related to IL-18 are registered in NCBI.)
Future Perspectives
Exploring the mechanisms and clinical applications of IL-18 in inflammatory diseases can provide critical insights for the diagnosis and treatment of related conditions. The measurement of IL-18 concentrations and modulation of IL-18 signaling pathways may emerge as novel therapeutic targets in the future.
In summary, IL-18 plays a significant role in the pathogenesis and treatment of inflammatory diseases. A deeper understanding of its biological functions and clinical implications will advance the diagnosis and management of related conditions.
1.Emily L. et al.Biological and clinical roles of IL-18 in inflammatory diseases.Nature Reviews Rheumatology20,pages33-47(2024).
2.Bossaller, L. et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189, 5508–5512 (2012).
3. Omoto, Y. et al. Granzyme B is a novel interleukin-18 converting enzyme. J. Dermatol. Sci.59, 129–135 (2010).
4. Sugawara, S. et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 167, 6568–6575 (2001).
35. Omoto, Y. et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J. Immunol. 177, 8315–8319 (2006).
5. Aguilar, C. et al. Characterization of Crohn disease in X-linked inhibitor of apoptosis-deficient male patients and female symptomatic carriers. J. Allergy Clin. Immunol. 134, 1131–1141.e9 (2014).
6.Canna,S.W. et al. Brief report: alternative activation of laser-captured murine hemophagocytes. Arthritis Rheumatol. 66, 1666–1671 (2014).
7. Billiau, A. D., Roskams, T., Van Damme-Lombaerts, R., Matthys, P. & Wouters, C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood 105, 1648–1651 (2005).
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA040236 | IL-18BP Protein, Human | Host : Human | $72 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA010574 | IL-18BP His Tag Protein, Human | Host : Human | $317.40 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA010587 | IL-18BP Fc Chimera Protein, Human | Host : Human | $317 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA040226 | IL-18 Protein, Rat | Host : Rat | $296 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040195 | IL-18 Protein, Mouse | Host : Mouse | $176 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA040111 | IL-18 Protein, Human | Host : Human | $164 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated |




