The MOG-induced EAE animal model: An effective tool for studying autoimmune diseases.
Introduction
Multiple Sclerosis (MS) is a classic autoimmune disease whose pathological mechanisms are not yet fully understood. To further investigate and comprehend the pathogenesis of MS, researchers widely employ the Experimental Autoimmune Encephalomyelitis (EAE) animal model. In these models, Myelin Oligodendrocyte Glycoprotein (MOG) is utilized as an immunogen to induce autoimmune lesions, mimicking the progression of MS.
Research has reported that MOG is the only central nervous system (CNS) myelin protein component capable of eliciting both demyelinating antibody responses and T-cell responses. Therefore, the MOG-induced EAE model provides a better research platform for elucidating the pathogenesis of MS/EAE.
Establishment of the MOG-Induced EAE Animal Model
MOG is a glycoprotein located on the surface of myelin and exhibits high immunogenicity. By using MOG as an antigen to trigger an immune response through immunization, the EAE animal model can be established. The selection of MOG is based not only on its abundant expression in myelin but also on its ability to effectively induce autoimmune lesions across multiple species.
The MOG-induced EAE model is characterized by rapid onset, ease of operation, and high reproducibility. These features enable researchers to better simulate the immunological and pathological characteristics of MS under controlled experimental conditions, providing an effective tool for disease research.
Case Study
Engineered MBP-Specific Human Tregs Ameliorate MOG-Induced EAE via IL-2-Triggered Effector T Cell Suppression
To further demonstrate the bystander suppression function of Ob2F3 Tregs in vivo, the MOG-induced Experimental Allergic Encephalomyelitis (EAE) model was employed in human HLA DR15 transgenic mice as an animal model for MS. On day 7 post-MOG immunization, human Ob2F3 Tregs were intravenously injected, by which time CNS inflammation was well established. Although these cells are xenogeneic to the mouse host, Ob2F3 Tregs significantly reduced the mean and cumulative disease scores compared to PBS and 17195 Treg-infused mice in the MOG-induced EAE model (Figures A and B). Histological analysis confirmed CNS migration of Ob2F3 Tregs in the brain and spinal cord two days post-injection (Figure C), with 17195 Tregs showing lower migration levels on the same day. Importantly, the intensity of hCD4-PE was significantly higher in both tissues of Ob2F3 Treg-injected mice compared to those injected with non-specific 17195 TCR Tregs (C). By day 17, the injected Ob2F3 or 17195 human Tregs were no longer detectable, likely due to xenogeneic recognition in the brain or spinal cord tissues. Furthermore, histopathological analysis of spinal cord tissues revealed reduced inflammation in Ob2F3-treated mice on day 17 compared to the 17195 or PBS groups, consistent with the observed decrease in EAE scores in Figure A (Figure D). To confirm the potential accumulation of infused human Tregs, slides were further stained with anti-human CD4 and anti-mouse CD4 antibodies: perivascular cells were predominantly mouse CD4 T cells (data not shown). From these in vivo results, we conclude that monoclonal-focused MBP-specific Ob2F3 Tregs can infiltrate inflamed CNS tissues and ameliorate the pathogenesis of MOG-induced encephalomyelitis through bystander suppression mechanisms in the local environment.
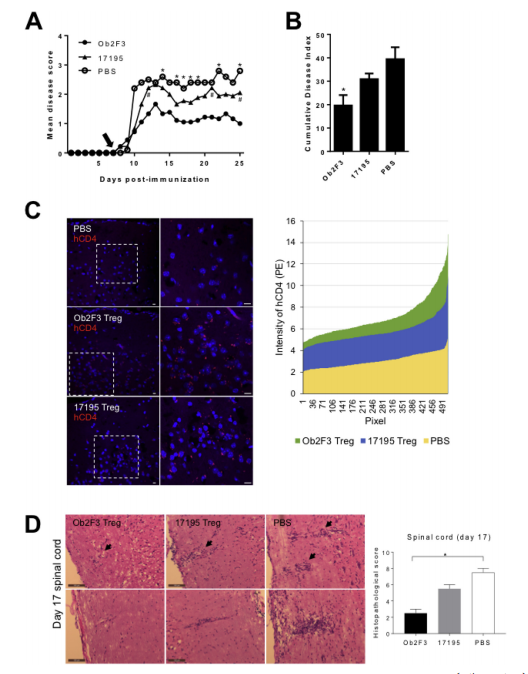
Ob2F3 Tregs Ameliorate MOG35-55-Induced EAE in Humanized HLA DR15 Mice.EAE was actively induced in male and female HLA DR15 mice (4.5-7.5 months old) using pMOG35-55/CFA/PT. On day 7 post-immunization, mice were intravenously injected with 2×10^6 engineered MBP-specific Ob2F3 human Tregs (Ob2F3) or control 17195 human Tregs (17195, n=9). An additional control group was injected with PBS (n=5). EAE symptoms were scored daily. The mean clinical score (A) and cumulative disease index (B) of EAE are shown. Data are from two independent experiments with similar results and are presented as mean ± SEM. Significance between groups was analyzed by Mann-Whitney U test. *p<0.05, Ob2F3 group vs. PBS group; #p<0.05, Ob2F3 group vs. 17195 group. (C) Immunofluorescence images of PE-conjugated anti-human CD4 (red) antibodies in horizontal brain cryosections of injected mice on day 9. Nuclei were counterstained with DAPI (blue). Original magnification 40×, with insets re-captured within the magnification range (right). Scale bar, 10 μm. The right panel shows the hCD4 intensity distribution of magnified images measured by ZEN2012 software, arranged in ascending order. (D) Representative H&E-stained spinal cord sections from PBS, 17195 Treg, and Ob2F3 Treg-injected mice, injected on day 9 and harvested on day 17. Arrows indicate perivascular brain regions. Original magnification 20×. Scale bar, 100 μm.
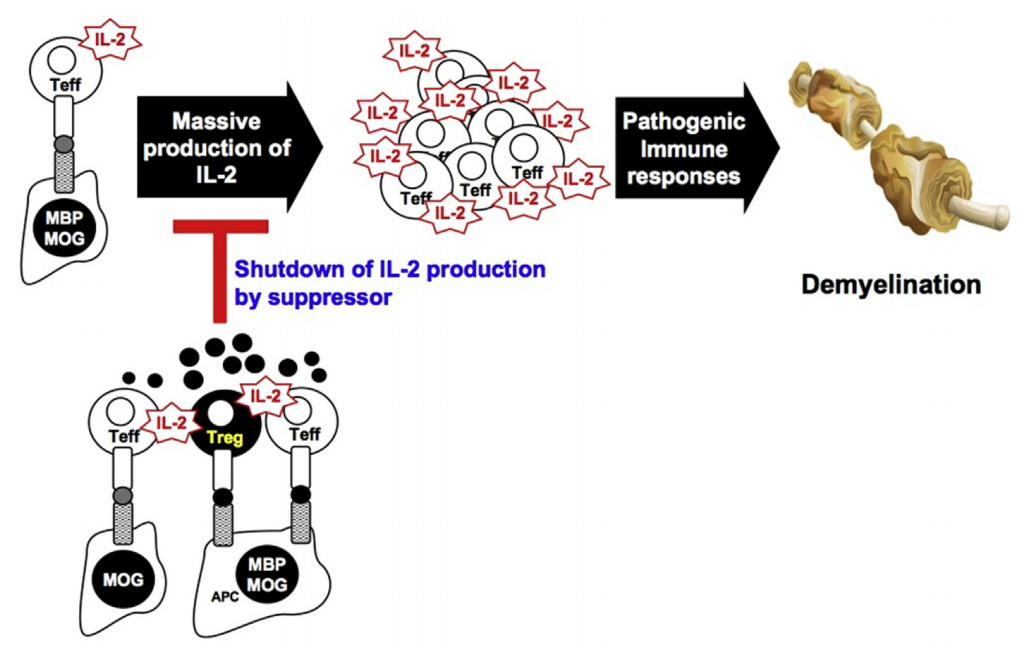
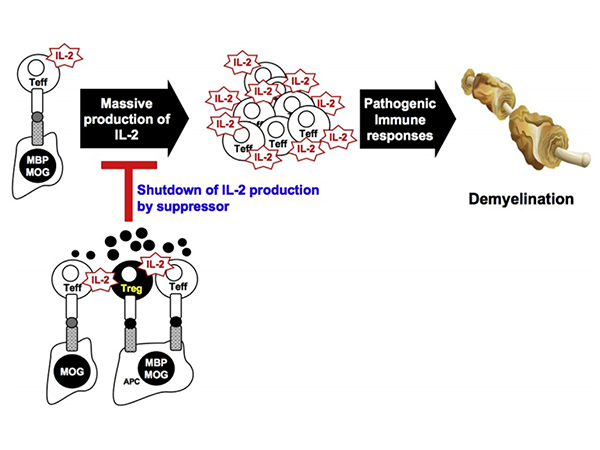
Non-Contact Suppression Mechanism of MBP-Specific Engineered Tregs on MOG-Specific Effector T Cells.MOG- or MBP-specific T cell clones are initially activated by IL-2 production in response to MOG or APC-presented MBP. IL-2 produced by effector cells provides a strong secondary signal during the expansion of MOG- or MBP-specific T effectors in lymph nodes and inflamed CNS, leading to pathogenic demyelination. By infusing MBP-specific monoclonal Tregs, these engineered Tregs are activated upon presentation of MBP p85-99 peptide and IL-2 secreted by T effectors, either shared by APCs or separated at proximal distances. Activated Tregs subsequently secrete soluble inhibitory factors, thereby achieving non-contact bystander suppression in the local inflammatory environment by suppressing further proliferation of pathogenic T effectors through the dephosphorylation of Stat5, a key downstream signaling pathway of IL-2.
Applications of the MOG-Induced EAE Animal Model in Research
1. Pathophysiological Research on Multiple Sclerosis (MS): The MOG-induced EAE model is widely used to study the pathogenesis, pathophysiological processes, and potential treatments for MS. By simulating the immunological and neuropathological features of MS, researchers can evaluate the efficacy of various therapeutic strategies.
2. Research on Immunomodulatory Therapies: The MOG-EAE model can be used to assess the effects of different immunomodulatory therapies, such as immunosuppressants, immunomodulators, and biologics. These therapies may help reduce disease severity or delay progression.
3. Drug Screening and Evaluation: Researchers can utilize the MOG-EAE model for drug screening and evaluation to discover new treatments or assess the efficacy of existing drugs. These drugs may include immunomodulators, neuroprotective agents, and anti-inflammatory drugs.
4. Research on Immune Cells and Signaling Pathways: The MOG-EAE model can be used to study immune cell types (e.g., T cells, B cells, macrophages) and related signaling pathways involved in immune responses. This helps elucidate the pathogenesis of MS and other autoimmune diseases.
5. Evaluation of Neuroprotective Strategies: In addition to immunomodulatory therapies, the MOG-EAE model can be used to evaluate the effectiveness of neuroprotective strategies, such as the application of neurotrophic factors and neural repair therapies.
Conclusion
The MOG-induced EAE animal model provides an essential experimental tool for research on Multiple Sclerosis, facilitating a deeper understanding of the disease's pathogenesis. However, it is important to note that any animal model has its limitations. Researchers should consider both the advantages and limitations of this model to accurately interpret experimental results.
1.Kim,Yong,Chan,etal.Engineered MBP-specifific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effffector T cells.JournalofAutoimmunity,2018.
2.KrishnamoorthyG.,LassmannH.,&WekerleH(2006).Experimentalmodelsofspontaneousautoimmunediseaseinthecentralnervoussystem.JournalofMolecularMedicine,84(5),416-428.
3.OliverA.R.,LyonG.M.,&RuddleN.H(2003).RatandhumanmyelinoligodendrocyteglycoproteinsinduceexperimentalautoimmuneencephalomyelitisbydifferentmechanismsinC57BL/6mice.JournalofImmunology,171(1),462-468.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA010433 | MOG His Tag Protein, Mouse | Host : Mouse | $580 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA010471 | MOG(1-125) Protein, Human | Host : Human | $2,560 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA080263 | MOG(35-55), Human | Host : Human | $110 |




