Exploring the mRNA Vaccine Production Process: The Central Role of Enzymatic Products

Introduction
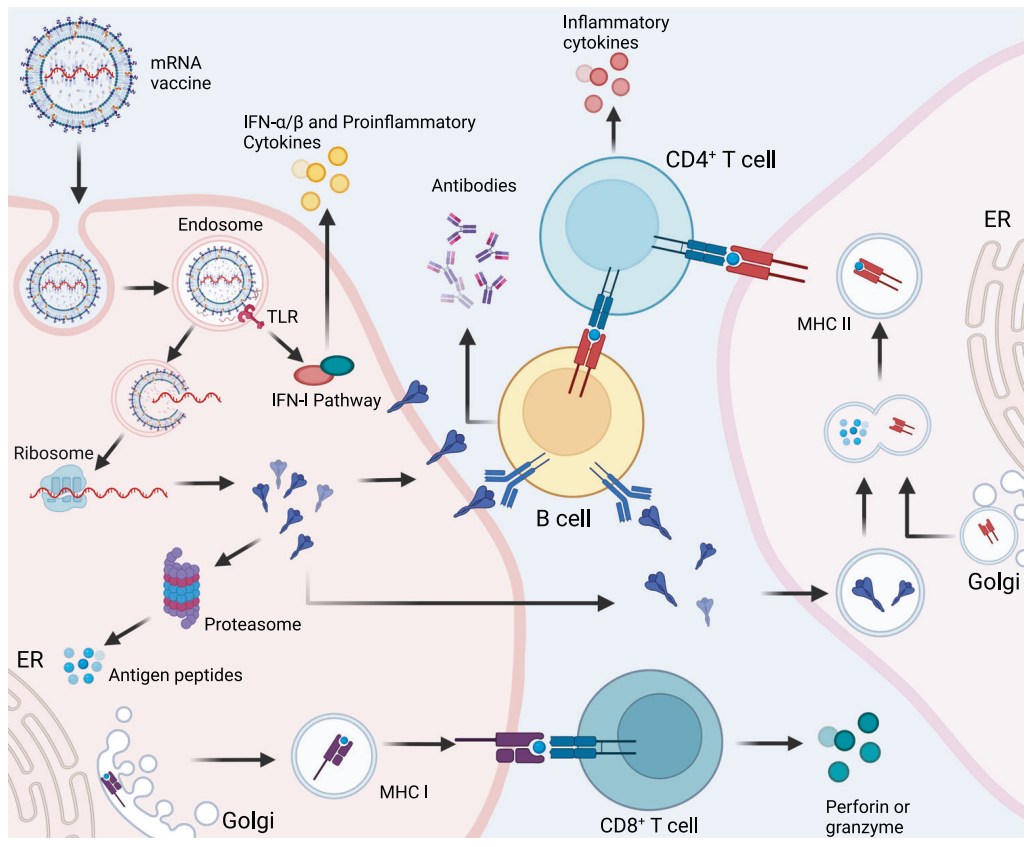
mRNA vaccines, as a significant achievement of modern biotechnology, have brought new hope to global public health due to their high efficiency and rapid response to pathogens. The core principle of mRNA vaccines lies in the delivery of mRNA encoding antigenic proteins into human cells, where it is directly translated into the corresponding antigenic proteins, thereby inducing a specific immune response in the body. Throughout this process, enzymatic products serve as critical tools, playing indispensable roles in multiple stages.
Mechanism of mRNA Vaccines
Source: SPRINGER NATURE
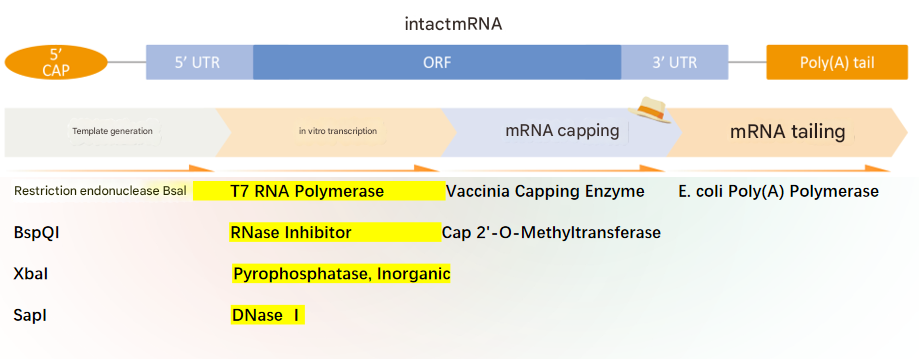
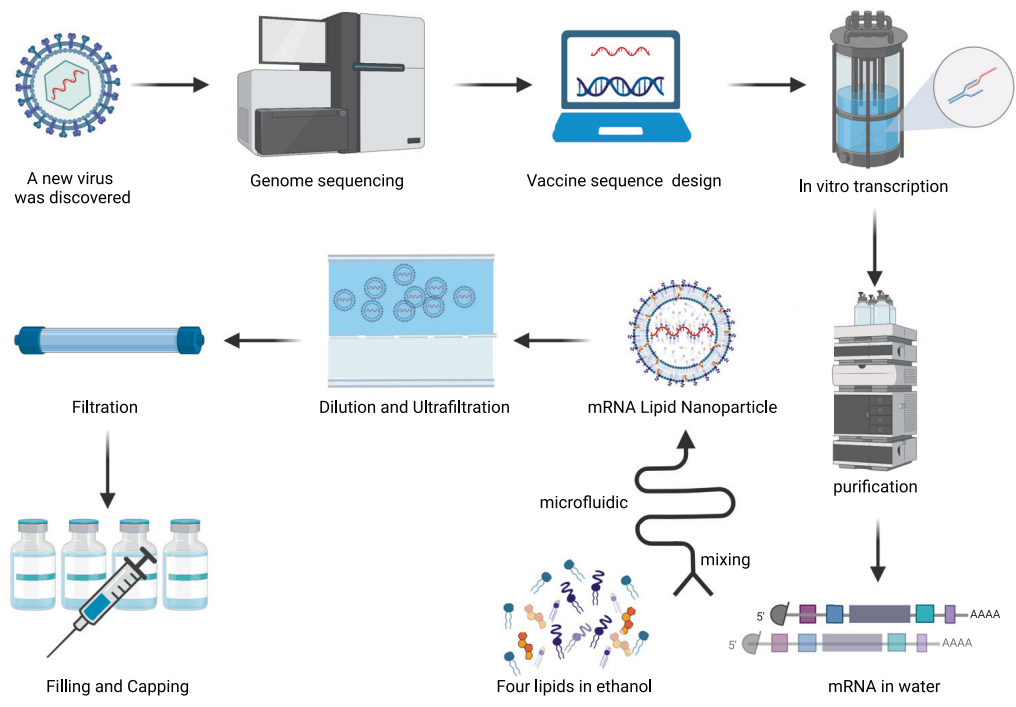
mRNA Vaccine Production Process
Source: SPRINGER NATURE
The design of mRNA vaccines depends on the identification of the target pathogen's antigen sequence. By defining the target antigen and optimizing its coding sequence, mRNA can be transcribed in vitro using RNA polymerase. The synthesized mRNA is purified through various processes and then encapsulated into mRNA-lipid nanoparticle (mRNA-LNP) complexes using microfluidic technology. Subsequently, LNP self-assembly is completed through dilution and ultrafiltration concentration. Finally, the mRNA vaccine is obtained after sterile filtration, filling, and capping.
Core Enzymes in mRNA Vaccine Production
Plasmid Linearization and DNA Template Preparation
The first step in mRNA vaccine production involves extracting viral DNA from the master cell bank. This DNA is then used to construct plasmids, which are DNA fragments carrying the target gene (i.e., the gene encoding the antigenic protein). To ensure efficient mRNA synthesis, the plasmid must be enzymatically cleaved to form a linearized double-stranded DNA template. In this step, the restriction enzyme Bsa I plays a crucial role. It specifically recognizes and cleaves sites in double-stranded DNA, linearizing the template plasmid and providing an ideal template for subsequent in vitro mRNA synthesis.
☆BsaⅠ Restriction Enzyme Catalog Number: UA070035

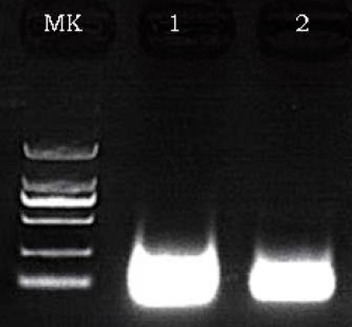
The results of 1μg pUC57 plasmid digestion separated under different quantity of BsaⅠ, The reaction was incubated for 60 minutes at 37°C, and 1% agarose gel was used for electrophoresis analysis after reaction.
M, marker;
Lane 1 1μg pUC57;
Lane 2 1μg pUC57 add 1U BsaⅠ
Lane 3 1μg pUC57 add 2U BsaⅠ

1μg (R: reducing condition, N: non-reducing condition).
In Vitro Transcription and Modification of mRNA
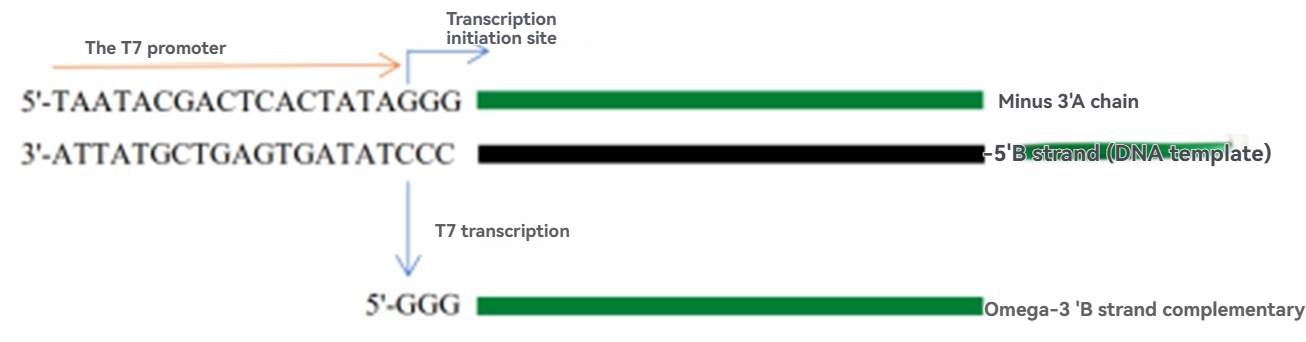
After obtaining the linearized DNA template, the next step is in vitro synthesis of mRNA. This process relies on T7 RNA polymerase, which specifically recognizes the T7 promoter sequence and catalyzes the synthesis of RNA complementary to the single-stranded DNA downstream of the promoter, using NTPs (nucleoside triphosphates) as substrates. T7 RNA polymerase is a key enzyme for in vitro transcription of mRNA, enabling the efficient production of high-quality mRNA in large quantities.
☆T7 RNA Polymerase Catalog Number: UA070073

The results of 1μg pUC57 plasmid digestion separated under different quantity of BsaⅠ, The reaction was incubated for 60 minutes at 37°C, and 1% agarose gel was used for electrophoresis analysis after reaction.
M, marker;
Lane 1 1μg pUC57;
Lane 2 1μg pUC57 add 1U BsaⅠ
Lane 3 1μg pUC57 add 2U BsaⅠ

1μg (R: reducing condition, N: non-reducing condition).
Transcription-Assisting Enzymes
In addition to T7 RNA polymerase, other enzymes such as Pyrophosphatase, Inorganic, Murine RNase Inhibitor, and DNaseⅠ play important roles in in vitro transcription.
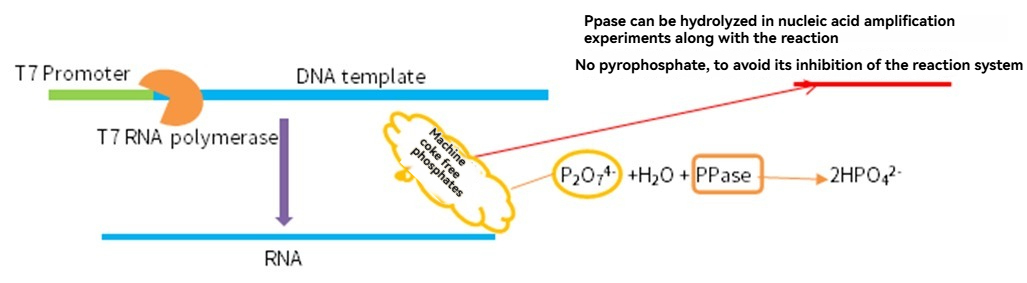
mRNA Capping and Modification
mRNA synthesis is not limited to transcription; it also requires a series of modifications to ensure functional integrity. Among these, Vaccinia Capping Enzyme and 2'-O-Methyltransferase add a Cap0 structure to the 5' end of the mRNA and convert it to a Cap1 structure, which is crucial for enhancing mRNA stability and translation efficiency. The Cap1 structure improves the translation efficiency of mRNA within cells, thereby increasing the expression levels of the protein encoded by the transfected mRNA.
We are continuously updating our range of related enzymatic products. For more information, please feel free to inquire.
Conclusion
In summary, enzymatic products play a pivotal role in the mRNA vaccine production process. From plasmid linearization and in vitro mRNA synthesis to mRNA purification, quality control, and final LNP encapsulation and delivery, each step relies on the efficient catalytic action of enzymes. Key enzymes such as restriction endonucleases, T7 RNA polymerase, Vaccinia Capping Enzyme, and 2'-O-Methyltransferase not only ensure the efficient production of mRNA vaccines but also enhance their quality and stability. This lays a solid foundation for the widespread application of mRNA vaccines globally.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA070036 | DNase Ⅰ | Host : Bovine Pancreatic | $91 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070037 | Murine RNase Inhibitor | Host : Rat | $40 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070038 | Pyrophosphatase, Inorganic | Host : Yeast | $47.20 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070035 | BsaⅠ | Host : Bacillus stearothermophilus | $36 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated |