ADC Drug Conjugation: Focusing on Glycosyl Conjugation Technology

Recent Advances
Introduction
Antibody-drug conjugates (ADCs), as a novel class of biopharmaceuticals, have garnered significant attention in the field of cancer therapy due to their unique mechanisms of action and remarkable therapeutic efficacy. ADCs typically consist of three components: a highly specific and high-affinity antibody, a highly stable linker, and a highly potent small-molecule cytotoxic drug. Among these, the drug conjugation technology is one of the critical factors influencing the performance of ADCs, determining key attributes such as the drug-to-antibody ratio (DAR), homogeneity, activity, tolerability, and stability.
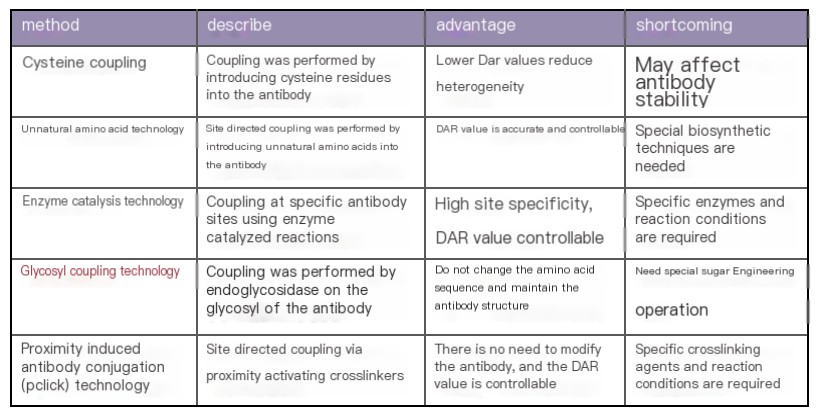
Overview of ADC Drug Conjugation Technologies
ADC drug conjugation technologies are broadly categorized into two types: non-site-specific conjugation (random conjugation) and site-specific conjugation. Non-site-specific conjugation primarily relies on lysine and cysteine residues. Lysine-based conjugation offers advantages such as simplicity and rapid reaction kinetics due to the high natural abundance and surface accessibility of lysine residues. However, it suffers from poor selectivity and heterogeneity, often resulting in ADCs with variable DARs and conjugation sites, which can affect the pharmacokinetic/pharmacodynamic (PK/PD) properties of the drug. Cysteine-based conjugation methods, on the other hand, involve the reduction of interchain disulfide bonds to expose free cysteine residues for linker conjugation, thereby reducing ADC heterogeneity and improving homogeneity. Nevertheless, challenges such as incorrect intrachain bridging remain to be addressed.
Site-specific conjugation technologies, which allow for better control of DAR and produce more homogeneous ADCs, have become a growing trend in conjugation method development. These technologies include the introduction of reactive cysteine residues (Thio-mab technology), disulfide re-bridging, incorporation of non-natural amino acids, enzymatic conjugation, glycosyl conjugation, and proximity-induced antibody conjugation (pClick) technology. Below, we will discuss these technologies in detail, with a focus on glycosyl conjugation technology.
Steps in Glycosyl Conjugation Technology
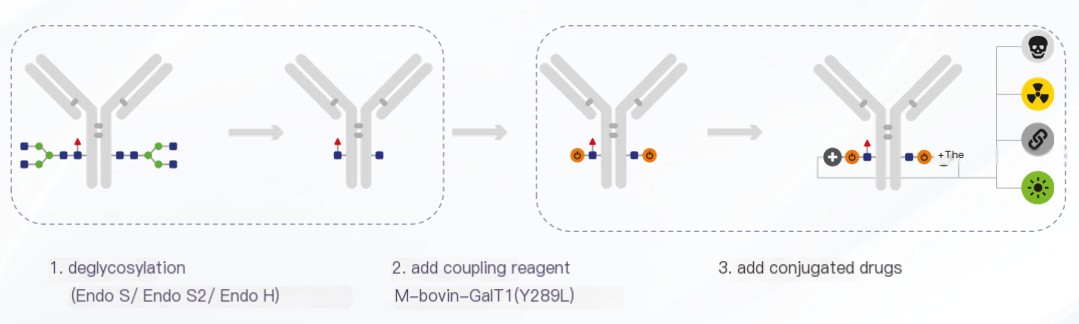
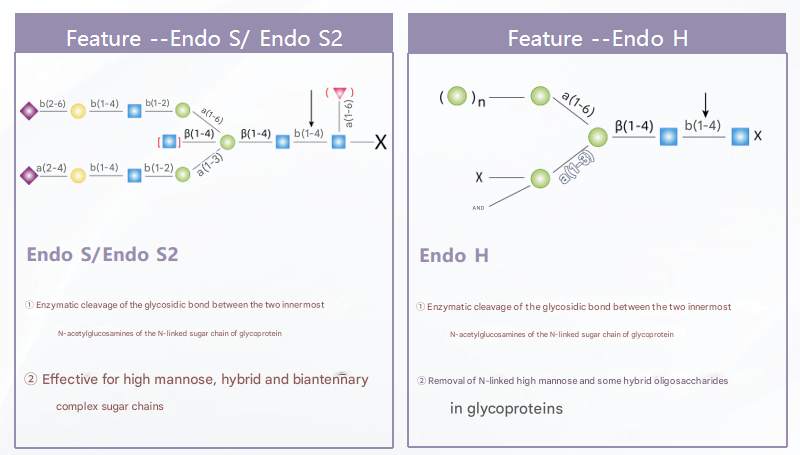
1.Deglycosylation:
The first step involves the removal of glycan chains from the antibody to expose the glycosylation sites.
2.Addition of Conjugation Reagents:
The enzyme M-bovin-GalT1(Y289L), which possesses glycosyltransferase activity, is used to label O-GlcNAcylated proteins with the N-azidoacetylgalactosamine (GalNAz) group.
Advantages of Glycosyl Conjugation Technology
-
High Homogeneity:
Glycosyl conjugation technology enables site-specific modification of glycans, resulting in a uniform distribution of drug linkers on the antibody and improving the homogeneity of ADCs. -
Enhanced Stability:
This technology avoids interference with antigen binding and antibody function, while the inherent stability and biocompatibility of glycan chains further enhance the stability of ADCs. -
Improved Safety:
Glycosyl conjugation reduces interference with antigen receptors, minimizing off-target effects and enhancing the safety profile of ADCs.
Conclusion
The conjugation technology used in ADCs is a critical determinant of their performance. Glycosyl conjugation technology, as a novel site-specific conjugation approach, offers significant advantages such as high homogeneity, enhanced stability, and improved safety, demonstrating broad application prospects in ADC development. With ongoing advancements and innovations in conjugation technologies, glycosyl conjugation is poised to play a pivotal role in the development and application of ADCs, providing more effective tools for cancer therapy.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA070081 | M-bovin-GalT1(Y289L) | Expression System : HEK293 | $1,000 |
| UA070055 | Endo S2 | Host : Streptococcus pyogenes | $1,000 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070039 | Endo S | Host : Streptococcus pyogenes | $270 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070042 | Endo H (MBP Tag) | Host : Streptomyces picatus | $96 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated | |||
| UA070040 | Endo H | Host : Streptomyces picatus | $70 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated |




