Ubiquitination: Decoding the "Molecular Tagging System" of Cellular Regulation and New Perspectives on Disease Associations
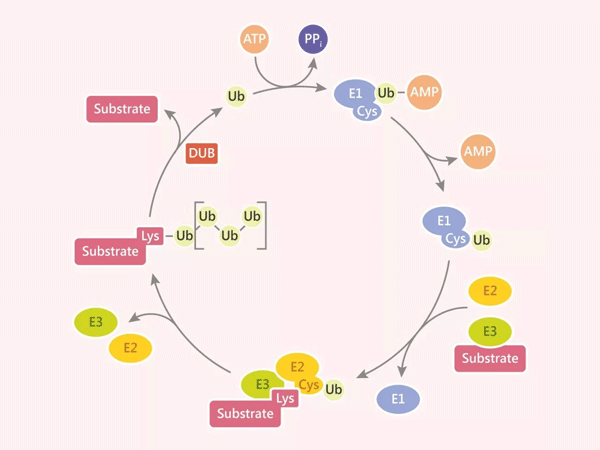
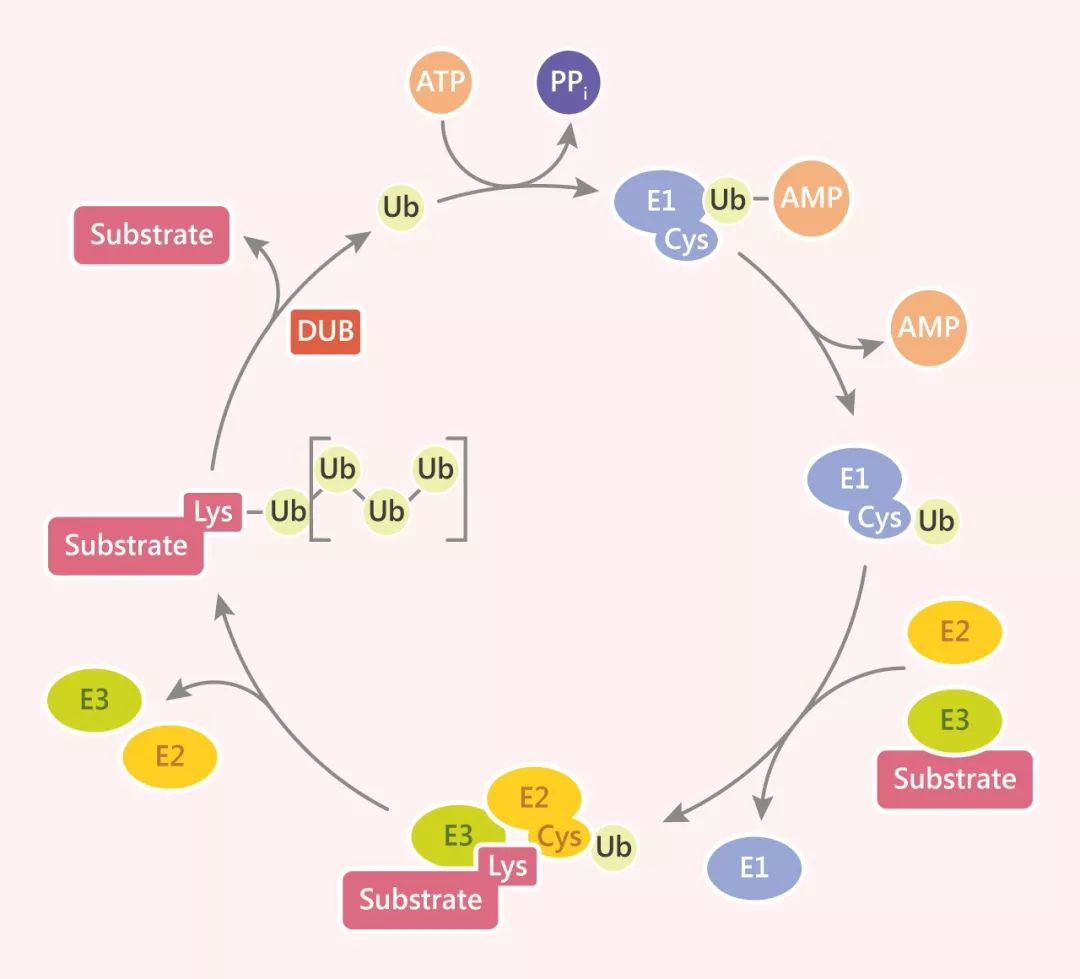
In the intricate regulatory network of cells, ubiquitination stands out as one of the most complex and multifunctional post-translational modifications. This process of covalently tagging target proteins with the small protein ubiquitin (Ub) acts like a dynamically rewritable "molecular tag" for proteins, not only determining their fate but also playing a central regulatory role in all dimensions of cellular life activities. From the precise progression of the cell cycle to the molecular mechanisms of DNA damage repair, from the development of cancer to the pathological processes of neurodegenerative diseases, the ubiquitination system weaves a complex regulatory network covering cellular homeostasis and disease occurrence with its unique diversity and multivalency.
一,Ubiquitination: A Molecular Revolution from Basic Mechanisms to Functional Diversity
The core of ubiquitination lies in the E1-E2-E3 enzymatic cascade, which attaches a 76-amino-acid ubiquitin molecule to the lysine residues of target proteins. This process is far from a simple "protein degradation signal"; instead, it endows target proteins with rich functional regulatory dimensions based on the length of the ubiquitin chain (mono-ubiquitination, multi-ubiquitination, poly-ubiquitination) and the linkage mode (8 lysine sites including Met1, K6, K11, and N-terminal methionine):
- Tag Type Determines Functional Output: K48-linked poly-ubiquitination typically marks proteins for proteasomal degradation, such as the negative regulator of p53, MDM2, promoting p53 degradation through K48 ubiquitination. K63-linked ubiquitination, on the other hand, is more involved in signal transduction and membrane trafficking, with EGFR endocytosis and sorting enhanced by K63 ubiquitin chains.
- Dynamic Reversible Regulatory Balance: Deubiquitinating enzymes (DUBs) like the USP family form a bidirectional regulation with the ubiquitination system, ensuring precise cellular responses in key processes such as proliferation and apoptosis. The imbalance of this dynamic equilibrium is often an important trigger for disease occurrence.
二,Core Hub of Cellular Regulation: Multidimensional Functional Analysis of Ubiquitination
1,Cell Cycle: Ubiquitination's Precise Timing Mechanism
In the "countdown" to cell division, two types of E3 ligases, SCF and APC/C, act as "molecular clocks" through specific ubiquitination modifications: SCF recognizes phosphorylated substrates (such as p27) to promote S phase progression, while APC/C degrades cyclins through K11 ubiquitination to drive mitosis. These spatiotemporally specific ubiquitination events ensure the highly ordered chromosome segregation and cell cycle transitions. Abnormalities such as APC/C dysfunction directly lead to genomic instability, a hallmark of cancer.
2,Receptor Internalization: Dynamic Terminator of Membrane Signals
The termination of signals from transmembrane receptors like EGFR relies on ubiquitination-mediated endocytosis and sorting: The Cbl family of E3 ligases catalyzes K63 mono-ubiquitination of EGFR, determining its entry into clathrin-dependent or -independent internalization pathways, thereby affecting signal duration and cellular responses. The failure of this "signal braking" mechanism, such as EGFR ubiquitination abnormalities leading to sustained activation, is a key driver in the development of various solid tumors.
3,DNA Damage Repair: The "Molecular Mender" of the Genome
In response to DNA damage, ubiquitination initiates the repair cascade through mono-ubiquitination modifications: K561 mono-ubiquitination of Fanconi anemia-related protein FANCD2 mediates its recruitment to damage sites and synergizes with BRCA1/2 to repair cross-linked DNA. Defects in this precise damage response network, such as mutations in the FANCD2 ubiquitination site, lead to chromosomal instability and significantly increase the risk of leukemia and squamous cell carcinoma.
4,Apoptosis: The Molecular Switch for Life and Death Decisions
Ubiquitination exhibits bidirectional regulation in apoptosis control: MDM2 degrades the tumor suppressor gene p53 through K48 ubiquitination, relieving cell cycle arrest and apoptosis induction. Meanwhile, apoptosis inhibitors IAPs ubiquitinate and degrade pro-apoptotic proteins Caspase, forming anti-apoptotic protection. The disruption of this balance, such as MDM2 overexpression leading to p53 dysfunction, is a classic mechanism by which tumor cells evade apoptosis.
三,Disease Associations: From Mechanism Abnormalities to New Therapeutic Targets
1,Cancer: Systematic Imbalance of the Ubiquitination Network
Ubiquitination dysregulation has multidimensional effects in tumorigenesis:
- Protein Stability Regulation: VHL gene deletion leads to the failure of HIF-1α ubiquitination degradation, promoting tumor angiogenesis. Abnormal activation of SCF-SKP2 degrades p27, driving cell cycle失控.
- Signal Pathway Activation: Mono-ubiquitination of K-RAS enhances its GTP-binding ability, activating the PI3K-AKT pathway. EGFR ubiquitination defects lead to sustained MAPK signal activation, promoting cell malignant transformation.
Breakthroughs have been made in drug development targeting the ubiquitination pathway, such as the application of the proteasome inhibitor bortezomib in multiple myeloma, and MDM2 inhibitors that restart the apoptosis pathway by relieving p53 inhibition.
2,Neurodegenerative Diseases: The Starting Point of Protein Homeostasis Collapse
In Parkinson's disease, abnormal ubiquitination of α-synuclein forms Lewy bodies, impeding proteasome function and triggering neuronal death. In Alzheimer's disease, hyperphosphorylation and ubiquitination of tau protein lead to neurofibrillary tangles, and decreased UPS system efficiency accelerates pathological progression. The site specificity of ubiquitination modifications (such as K63 ubiquitination of α-synuclein promoting aggregation) provides targets for developing precise intervention strategies, with small-molecule compounds targeting abnormal ubiquitination aggregates already entering preclinical studies.
四,Future Prospects: From Basic Analysis to Precise Intervention
Despite the revelation of ubiquitination's central role in cellular regulation and disease, many mysteries remain: the functional diversity of atypical ubiquitin chains (such as K11, K27), the cross-regulatory network of ubiquitination with phosphorylation/acetylation, and how to develop highly selective drugs targeting the substrate specificity of E3 ligases. With advancements in proteomic technologies and gene-editing tools, the ubiquitination system is moving from "basic mechanism research" towards "precision medical applications":
- Targeting E3 Ligases: Developing specific inhibitors like those for VHL and MDM2 to precisely restore tumor suppressor function.
- Regulating Deubiquitinating Enzymes: Rescuing tumor suppressor protein expression by activating USP family members or enhancing proteasome degradation efficiency by inhibiting DUBs.
- Multi-Omics Integration: Combining ubiquitinome profiling with single-cell sequencing to map disease-related ubiquitination dynamic landscapes and achieve personalized treatment strategies.
Conclusion: Decoding Molecular Tags, Opening New Dimensions of Precise Regulation
The discovery and research of the ubiquitination system have not only revolutionized our understanding of protein function regulation but also revealed the deep logic underlying cellular homeostasis maintenance and disease occurrence. From its initial recognition as a "protein degradation signal" to its current status as a "molecular operating system" covering multidimensional regulation, the story of ubiquitination is far from over. With technological advancements and in-depth mechanism analysis, this conserved system present in all eukaryotes, differing by only 3 amino acids, is leading us towards new frontiers in precise disease intervention. Perhaps, as its discovery journey suggests, each decryption of ubiquitination function is a key to unlocking the black box of cellular regulation, ultimately bringing disruptive breakthroughs in the treatment of major diseases like cancer and neurodegenerative disorders.




