Antibody-Drug Conjugates: How "Biological Missiles" for Precision Cancer Cell Sniping Are Rewriting Oncology Treatment
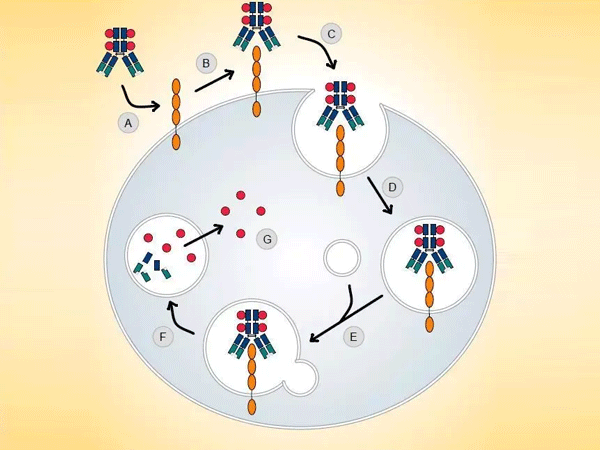
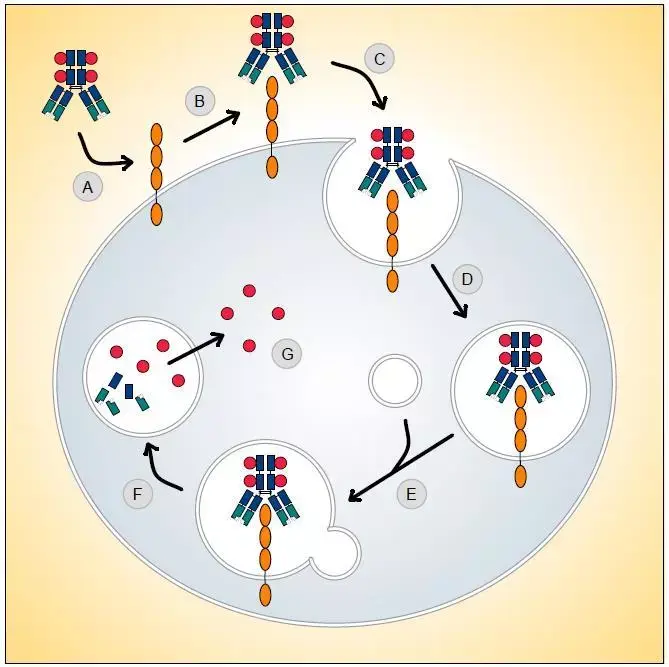
In the long journey of cancer treatment, achieving "precision strikes on cancer cells without harming innocents" has always been a core challenge in the medical community. The emergence of antibody-drug conjugates (ADCs) is like equipping drugs with a "guidance system," upgrading chemotherapy from "carpet bombing" to "precision missile strikes." This innovative therapy, which combines the targeting ability of antibodies with the killing power of cytotoxic drugs, is rewriting the landscape of cancer treatment with an average annual market growth rate of 40%, becoming the fourth pillar of cancer treatment after chemotherapy, targeted drugs, and immunotherapy.
一,ADC's "Trinity" Design: A Breakthrough in Pharmaceutical Engineering from Concept to Reality
The ingenuity of ADCs lies in their "missile-like" molecular architecture, where three core components work together to achieve efficient killing through "precision guidance - targeted detonation - safe release":
- Antibody "Guidance System": The "Biological GPS" Targeting Cancer Cells
Monoclonal antibodies (such as trastuzumab and pertuzumab), engineered through genetic modification, can precisely identify antigens highly expressed on the surface of cancer cells (such as HER2, Trop-2, Claudin 18.2). These antigens act like "unique house numbers" for cancer cells, guiding ADCs to accumulate in tumor tissues, achieving 10-100 times higher drug concentration in tumors compared to the systemic diffusion of traditional chemotherapy drugs.
- Toxin "Warhead": A New Generation of Efficient and Low-Toxicity Cellular Killers
Unlike traditional chemotherapy drugs (such as paclitaxel and doxorubicin), the small-molecule toxins carried by ADCs are specially designed:
- High Lethality: Toxins such as monomethyl auristatin E/F (MMAE/MMAF) and camptothecin derivatives (SN-38) are 100-1000 times more lethal than conventional chemotherapy drugs, requiring only 1-2 molecules to enter cells to induce apoptosis.
- Low Immunogenicity: Synthetic chemical modifications reduce toxin-induced immune responses, avoiding systemic inflammation.
- Linker "Safety Catch": The Key to Balancing Stability and Release Efficiency
The linker acts like a "safety device" for the missile, needing to meet dual requirements:
- Circulatory Stability: Remaining stable in the bloodstream to avoid premature toxin release that could damage normal cells (e.g., polyethylene glycol modification enhances water solubility).
- Microenvironment Responsiveness: Upon reaching the tumor, precisely releasing the toxin through pH sensitivity (tumor microenvironment pH 6.5-6.8), enzymatic cleavage (cathepsin B), or reduction (glutathione) mechanisms. The currently clinically dominant valine-citrulline (Val-Cit) linker achieves targeted release through lysosomal enzyme cleavage.
二,ADC's "Dimensional Reduction Strike" Advantage: Three Core Characteristics Reshaping Treatment Logic
1,Breaking Through Targeted Therapy Drug Resistance
When targeted drugs fail due to antigen mutations (such as EGFR T790M) or bypass activation of signaling pathways, ADCs can expand their killing range through the "bystander effect" – the released toxin can penetrate cell membranes and kill neighboring cancer cells that do not express the target antigen, making them particularly suitable for heterogeneous solid tumors (such as triple-negative breast cancer and non-small cell lung cancer).
2,Unlocking "Unduggable" Targets
ADCs demonstrate unique advantages against membrane proteins that are difficult to target with traditional targeted drugs (such as glycoproteins and adhesion molecules):
- Claudin 18.2: The world's first approved Claudin 18.2 ADC (Zolbetuximab) has rewritten gastric cancer treatment guidelines, extending median overall survival to 13.2 months in patients with high Claudin 18.2 expression.
- Trop-2: Sacituzumab govitecan targets Trop-2, achieving an objective response rate of 35% in triple-negative breast cancer, becoming the first approved Trop-2 ADC.
3,Constructing a New Paradigm of "Precision Chemotherapy"
The efficacy of ADCs is closely related to the "drug-to-antibody ratio" (DAR). By controlling the attachment of 2-8 toxin molecules per antibody, targeting is ensured while avoiding antibody structural damage. For example, in HER2-positive breast cancer, trastuzumab ADC (T-DXd) has a DAR of 8, showing a threefold increase in efficacy compared to traditional T-DM1 (DAR=3.5), with significantly reduced cardiac toxicity.
三,Global Market Explosion: From "Niche Innovation" to "Billion-Dollar Race"
Currently, 16 ADC drugs are on the global market, with a market size of 13.8billionin2023,expectedtoexceed60 billion by 2030. Three driving forces are accelerating industry transformation:
- Rapid Expansion of Indications
From hematological malignancies (such as CD19 ADC for B-cell lymphoma) to solid tumors (such as HER2 ADC covering gastric, lung, and breast cancers), ADC indications have covered 12 major cancer types. In 2023 alone, five new drugs were approved, including the first bispecific ADC (REGN1500/RG7802).
- Technological Platform Iteration and Upgrades
- Novel Linkers: The proportion of cleavable linkers (such as vc-MMAE) decreased from 60% in 2015 to 45% in 2023, with non-cleavable linkers (such as thioether linkers) gaining more favor due to stability advantages.
- Bispecific ADCs: Simultaneously targeting two antigens (such as EGFR/c-Met) to address tumor antigen heterogeneity, preclinical data showing a twofold increase in tumor accumulation efficiency.
- Genetically Engineered Antibodies: Modifying the Fc segment to reduce ADCC effects (such as IgG4 subtypes) and prolong drug half-life (such as disitamab vedotin with a half-life of 14 days).
- Rise of Chinese Innovation
Domestic companies like RemeGen (RC48), Heng Rui Medicine (SHR-A1811), and Kelun Biotech (SKB264) are leading ADC R&D. 35% of global ADCs in development are from China, covering popular targets like HER2, Claudin 18.2, and c-Met. Some products (such as RC48) have received FDA breakthrough therapy designation, opening a new journey for "Chinese innovative drugs going global."
四,Challenges and Future: From Technical Breakthroughs to Ecosystem Construction
1.Three Core Technical Bottlenecks
- Off-Target Toxicity: Side effects caused by low antigen expression in normal tissues (such as the 13% incidence of interstitial pneumonia with T-DXd), requiring optimization through bispecific targeting and spatiotemporal controllable release technologies.
- Drug Resistance Mechanisms: Including antigen downregulation (such as in HER2-low tumors), lysosomal dysfunction, and drug efflux pump activation, requiring combination with targeted drugs (such as mTOR inhibitors) or immunotherapy (PD-1 monoclonal antibodies) to overcome.
- Production Processes: Stringent CMC requirements for ADC uniformity control (DAR distribution) and stability testing (aggregation analysis), with only 12 companies globally currently having large-scale production capacity.
2.Future R&D Directions
- Multispecific ADCs: Simultaneously targeting tumor antigens and immune checkpoints (such as PD-L1/Her2 bispecific ADCs) to achieve dual effects of "targeted killing + immune activation."
- Precision Patient Selection: Establishing a three-dimensional biomarker system based on antigen expression levels (IHC score), tumor microenvironment (matrix metalloproteinase levels), and pharmacokinetics (ADC clearance rate), such as Kadcyla using HER2 IHC 3+ as a core enrollment criterion.
- Intelligent Design: Utilizing AI to predict antibody-antigen binding affinity (such as the DeepMAB platform) and toxin release kinetics, shortening the ADC R&D cycle from the traditional 5-7 years to 3-4 years.
Conclusion: ADCs Usher in the Era of "Precision Detonation" in Cancer Treatment
From Paul Ehrlich's concept of "magic bullets" in 1913 to the ADC global market breaking the billion-dollar mark in 2023, it has taken humanity a century to turn this dream into reality. This innovative drug, which integrates antibody targeting, toxin efficiency, and linker intelligence, has not only rewritten the treatment landscape for classic targets like HER2 but also unlocked the therapeutic potential of numerous "unduggable" targets.
With breakthroughs in bispecific ADCs, multi-toxin conjugation, and AI-assisted design, ADCs are evolving from "precision-guided missiles" to "smart cruise missiles" – capable of both recognizing the "unique identifiers" of individual cancer cells and dynamically adjusting attack strategies based on the tumor microenvironment. In the future, with the refinement of precision screening systems and the maturation of combination therapies, ADCs are expected to form synergistic networks with immunotherapy and gene therapy, jointly constructing a new ecosystem of "personalized precision oncology." In this prolonged battle against cancer, ADCs are not just a drug but a key to unlocking precision and intelligence in cancer treatment, leading humanity towards the ultimate goal of "curing cancer."




