Green Fluorescent Protein: A Bioluminescent Revolution from the Deep Sea to the Lab
In the brilliant starry sky of life sciences, green fluorescent protein (GFP) stands out as the most dazzling "scientific lighthouse." Originating from deep-sea anemones, this magical protein has given scientists the "X-ray vision" to see inside cells for the first time with its inherent fluorescent magic. From its initial discovery in 1962 to the evolution of a colorful fluorescent family today, the history of GFP is not only a chronicle of technological innovation but also a milestone in modern biological research.
- Deep-Sea Light Seeking: The Past and Present of GFP
In 1962, Japanese scientist Osamu Shimomura discovered the mysterious green glow in the Aequorea victoria jellyfish during his research, unveiling GFP. In 1994, Martin Chalfie transferred the GFP gene into the nematode Caenorhabditis elegans. When the Science cover first showcased the glowing neurons, biological research officially entered the "visualization" era. This protein, with a diameter of only 3.2 nanometers, is woven from 238 amino acids. Its core is a "fluorescent garland" composed of serine, tyrosine, and aspartate, converting ultraviolet light into 509nm green fluorescence through conjugated resonance, like a miniature lantern inside cells.
- Technological Iteration: From Monochromatic Glow to Spectral Feast
The limitations in brightness and stability of wild-type GFP gave birth to a decades-long "protein transformation revolution":
- Brightness Innovation: The Birth of EGFP
The S65T single-point mutation in 1994 was a stroke of genius, simplifying GFP's excitation peak to a single peak at 488nm and enhancing its fluorescence intensity several times, resulting in the widely used enhanced GFP (EGFP). Its spectral characteristics are highly compatible with the classic fluorescent dye FITC, making it a golden partner for flow cytometry and fluorescence microscopy.
- Dynamic Tracking: Short-Lived dEGFP
To address the issue of long protein half-life, scientists fused the PEST degradation sequence of the mouse ODC gene with EGFP, creating the unstable dEGFP. This made "dynamic movies" of real-time gene expression monitoring possible, making the synthesis and degradation processes of intracellular proteins clearly visible.
- Color Empire: From Monochrome to Rainbow
The magic of genetic engineering has transformed GFP into a splendid spectral family. By replacing key amino acids, EYFP emits a yellow-green glow, ECFP shines with cyan fluorescence, and EBFP emits deep blue light. These "fluorescent chameleons" support multicolor labeling techniques, allowing scientists to track multiple proteins simultaneously, as if equipping cells with a "color navigation system."
- Application Breakthrough: Unlocking New Dimensions in Life Sciences
The emergence of GFP has completely rewritten the paradigm of biological research, with applications covering all scales from molecules to living organisms:
- "Real-Time Monitor" of Gene Expression
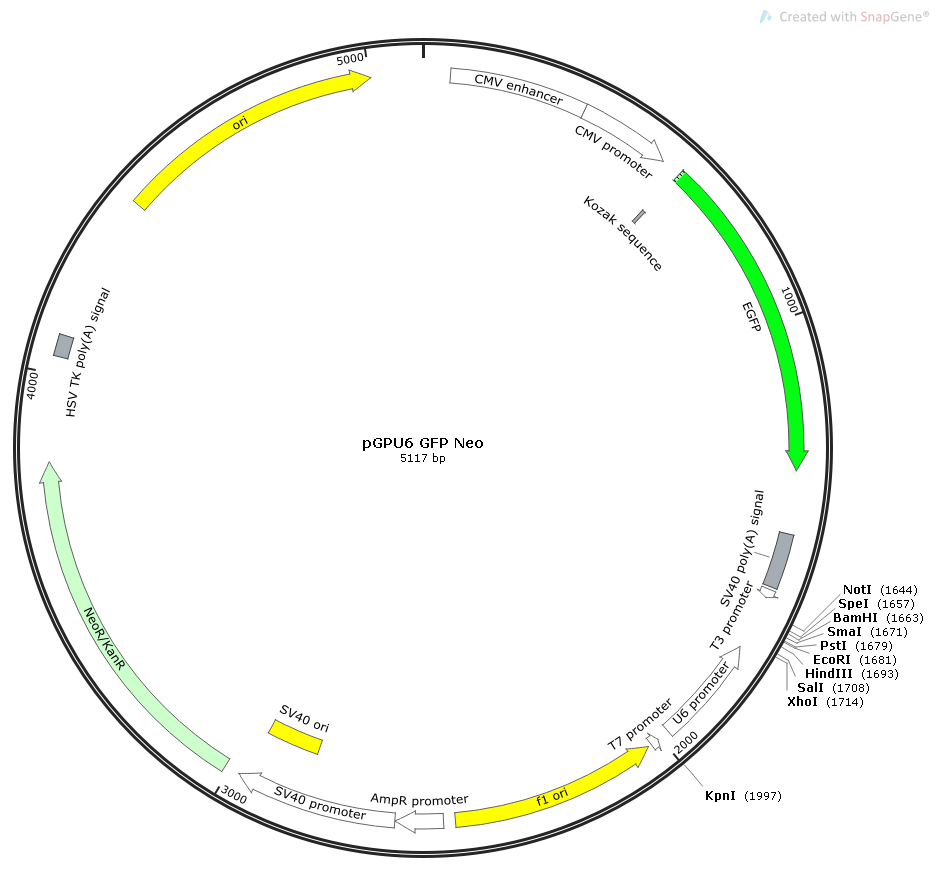
Fusing GFP with target genes is like labeling proteins with a glowing tag. In the R26-CAG-EGFP tool mouse constructed by Nanjing Biomedical Research Institute, green fluorescence precisely indicates gene expression in tissues such as the brain, kidney, and heart, providing a complete view of cell proliferation and differentiation during embryonic development.
- "Positioning Navigator" for Proteomics
Utilizing GFP's subcellular localization properties, scientists have observed "invisible" processes such as mitochondrial dynamic fusion and endoplasmic reticulum protein transport for the first time. When GFP-labeled membrane proteins flicker on the cell membrane, the spatiotemporal code of cell signaling transduction is being gradually deciphered.
- "Fluorescent Road Sign" for In Vivo Research
In tumor models, GFP-labeled cancer cells help track metastasis pathways. In neuroscience, GFP illuminates the neuronal networks of C. elegans, unveiling the mysteries of neural signal transmission. Even in embryonic development research, GFP allows scientists to observe organ formation in live zebrafish for weeks.
- "Precision Ruler" for Drug Development
Through GFP reporter gene systems, the regulatory effects of drugs on specific proteins can be quantified in real-time. For example, in screening anticancer drugs, changes in GFP fluorescence intensity directly reflect the expression levels of target proteins, significantly accelerating the new drug development process.
四,Future Outlook: From Tool to Therapy
Today, the GFP family has far surpassed the scope of a single labeling tool:
- Multiphoton Imaging: Long-wavelength variants reduce light damage and support deep-tissue in vivo imaging, making 3D reconstruction of neuronal networks in mouse brains a reality.
- Fluorescence Resonance Energy Transfer (FRET): By transferring energy between GFP and receptor proteins, real-time monitoring of intracellular molecular interactions, such as dynamic changes in GPCR signaling pathways, is achieved.
- New Therapeutic Directions: Utilizing GFP's light-activation properties to develop light-controlled gene expression systems, providing new ideas for precise gene therapy.
From the survival strategy of deep-sea organisms to the "star molecule" in the lab, the story of GFP is a perfect symphony of scientific discovery and technological innovation. When we gaze at those fluorescent dots under the microscope, we see not only the trajectories of proteins but also the wise glow of human exploration of the essence of life. With technological advancements, GFP and its variants will continue to serve as "bioluminescent probes," illuminating unknown corners within cells and leading us into a new era of precise decoding of the code of life.
This luminous revolution, which began in the deep sea, continues to write legends – each flicker of fluorescence is a prelude to scientific breakthroughs, illuminating the next unknown biological realm.




