Protein Purification Marvel: How Flag Tags and Antibodies Serve as the "Positioning Navigators" in Scientific Research
In the microscopic world of molecular biology, each protein is like a ship in the vast ocean, and Flag tags act as "signal lighthouses" equipped for them, enabling scientists to precisely locate, capture, and study these "biological messengers." As the golden duo for protein purification and detection, the combination of Flag tags and their specific antibodies is becoming an indispensable tool in modern life science research with its efficient and convenient advantages.
- Flag Tags: Octapeptide-Built "Protein Positioning System"
(1) Design Principle: Artificially Synthesized Affinity Code
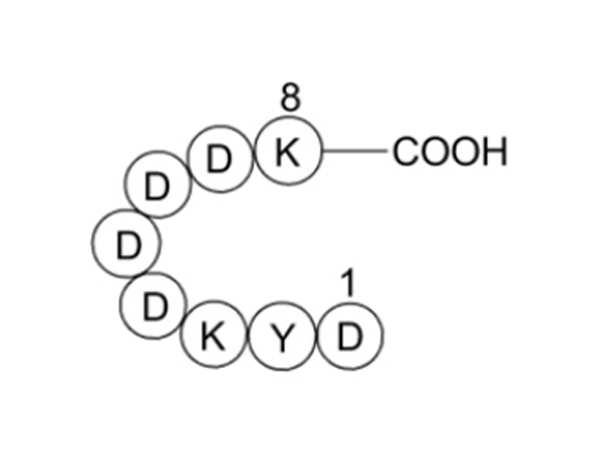
The Flag tag, composed of 8 amino acids (DYKDDDDK), is a masterpiece of protein engineering:
- Antigenic Core: The second tyrosine (Tyr), an aromatic amino acid, is a key site for antibody recognition. The negatively charged aspartates (Asp) on both sides form a highly polar microenvironment, significantly enhancing antigen-antibody binding.
- Hydrophilic Structure: The C-terminal pentapeptide sequence (DDDDK) constitutes an enterokinase cleavage site, allowing precise excision of the tag after purification to restore the natural protein and avoid interference with its function.

(2) Technical Advantages: Big Energy in a Small Tag
Compared to traditional affinity tags, the Flag system demonstrates unique advantages:
- Miniature Size, Versatile Function: A short peptide encoded by just 24 bases, it can be flexibly fused to the N- or C-terminus of the protein without affecting the target protein's conformation and activity, suitable for expression in all systems from bacteria, yeast to mammalian cells.
- Mild Purification, Activity Preservation: Achieves non-denaturing purification using antibody affinity chromatography without extreme conditions, ensuring the functional integrity of active molecules like enzymes and membrane proteins.
- Bidirectional Applications, Detection Assurance: Supports molecular detection methods such as Western Blot and ELISA, and can capture interacting proteins via immunoprecipitation, becoming a "multifaceted tool" for proteomic research.
(3) Tag Excision: Precisely Removing the "Navigation Device"
With specific proteases, Flag tags can be "tracelessly disassembled":
- Enterokinase Preferred: Recognizes the DDDDK sequence, leaving only 4 amino acids after cleavage. It is the gold standard for N-terminal tag excision, with Hosfield experiments confirming its cleavage efficiency can reach over 80%.
- Multi-Enzyme Options: Thrombin, Factor Xa, etc., provide alternative solutions, adapting to different experimental scenarios. For example, thrombin cleavage leaves two amino acids, suitable for studies with lenient terminal requirements.
- Anti-Flag Antibodies: "Precision Missiles" from Recognition to Purification
(1) Antibody Family: Iterative Upgrades of Three Generations
Specific antibodies targeting Flag tags have formed a "three musketeers" with complementary functions:
| Antibody Type | Recognition Characteristics | Application Scenarios | Advantageous Features |
|---|---|---|---|
| M1 | N-terminal specificity, Ca²+ dependent | Purification of fusion proteins without extra N-terminal amino acids | Early classic tool, requiring strict N-terminal exposure conditions |
| M2 | Pan-positional recognition, Ca²+ independent | Universal detection and purification (N-terminal / C-terminal / Met tag-containing) | High compatibility, supporting complex fusion protein systems |
| M5 | High affinity for Met-Flag sequences | Detection of cytoplasmically expressed proteins | Excellent affinity for N-terminally Met-modified proteins |
The M2 antibody stands out, recognizing Flag proteins with extra N-terminal amino acids or C-terminal fusions without the need for metal ions, becoming the most widely used "all-rounder."
(2) Preparation Process: Precision Sniper from Antigen to Antibody
- Antigen Construction: Preparing high-immunogenicity complexes by coupling Flag peptides to carrier proteins like bovine serum albumin (BSA) via carbodiimide methods.
- Hybridoma Technology: Screening high-titer hybridoma cell lines after mouse immunization, and purifying ascites using caprylic acid-ammonium sulfate to obtain high-specificity monoclonal antibodies.
- Functional Validation: Determining antibody titer, subtype, and cross-reactivity through ELISA, immunoblotting, etc., to ensure recognition accuracy.
(3) Diverse Applications: Unlocking New Dimensions in Protein Research
- "Magic Magnetic Beads" in Purification: One-step purification with anti-Flag affinity gels/magnetic beads achieves 10³-10⁴-fold enrichment efficiency. For example, Biolinkedin's L-1011 magnetic beads can quickly capture target proteins from complex lysates, greatly simplifying the purification process.
- "Molecular Hooks" for Interaction Studies: Combining immunoprecipitation technology with Flag tags to efficiently "fish out" interacting proteins, often used to dissect signaling pathways (e.g., membrane receptor dimerization studies), becoming a core tool for mapping protein interaction networks.
- "Location Probes" for Functional Analysis: Inserting tags into different domains and using antibody cross-linking to simulate ligand activation, studying membrane receptor signal transduction, such as triggering receptor aggregation to replace natural ligands and reveal the "black box" mechanisms of intracellular signal transmission.
- Real-World Scenarios: Full-Chain Applications from Basic Research to Drug Development
(1) Recombinant Protein Production
In antibody drug development, Flag tags assist in the expression monitoring and purification of monoclonal antibodies, ensuring antibody activity and yield. In enzyme production, non-denaturing purification preserves enzyme activity, providing highly active biocatalysts for industrial catalysis.
(2) Cell Signaling Research
Using M2 antibodies to detect Flag-tagged GPCR receptors on the cell membrane and track their internalization process after ligand binding. Capturing phosphorylated Flag proteins via immunoprecipitation to dissect cascade reactions in signaling pathways like MAPK.
(3) Protein Interactomics
Constructing Flag tag fusion libraries combined with mass spectrometry analysis for high-throughput screening of interaction networks between viral proteins and host cells, such as identifying host receptors for the SARS-CoV-2 spike protein, providing targets for antiviral drug design.
Conclusion: The Flag System – A "Swiss Army Knife" for Scientific Challenges
Since its first report by Hopp et al. in 1988, the Flag tag and antibody system has been in use for over three decades, consistently ranking among the top protein research tools. Its compact design and mild yet efficient characteristics not only simplify experimental procedures but also break through the application limitations of traditional tags, making complex protein manipulations "victorious."
In the era of precision medicine, when scientists need to target proteins from vast numbers or dissect molecular interactions in complex systems, the Flag system acts as a reliable "molecular guide," using precise positioning and efficient capture to help researchers erect clear "road signs" in the fog of life sciences. With technological iterations, this classic tool is continuing to integrate with cutting-edge technologies like CRISPR and single-cell sequencing, writing new chapters in protein research. Whether it's protein purification in basic laboratories or antibody production in the pharmaceutical industry, the combination of Flag tags and antibodies continues to prove that good scientific tools never let "setting the Flag" become empty talk but rather make every scientific goal achievable with "victory under the flag."




