From Cell Killers to Immune Activators: The Three-Generation Evolution and Future Breakthroughs of ADC Payloads
In the precision anticancer system of antibody-drug conjugates (ADCs), the payload acts like the "warhead" of a missile, with its lethality and specificity directly determining the therapeutic effect. From the initial "extensive attacks" borrowing chemotherapy drugs to today's "smart guidance" focusing on DNA-damaging agents and immunomodulators, payloads have undergone three generations of technological innovation, evolving from single cytotoxic molecules to "multifunctional weapons" integrating multiple mechanisms. This article will analyze how this core component is reshaping the anticancer landscape of ADC drugs from the perspectives of mechanism of action, innovation paths, and future directions.
- The "Ideal Weapon" Portrait of Payloads: Six Golden Rules for Precision Killing
The ideal payload must possess both "killer instinct" and "precision traits":
- Ultimate Toxicity: Picomolar (pM) cytotoxicity, ensuring that a small number of molecules can induce cancer cell apoptosis.
- Low Immunogenicity: Avoiding antibody responses and prolonging drug circulation time.
- Stability Advantage: Maintaining structural integrity in the bloodstream and responding to the microenvironment for release after reaching the tumor.
- Modifiability: Reserving coupling sites without affecting drug efficacy while being compatible with linker design.
- Bystander Effect: Killing neighboring cancer cells that do not express the antigen, addressing tumor heterogeneity.
- Intracellular Targeting: Acting on targets located inside the cell, avoiding interference from the extracellular environment.
These characteristics serve as "weapon acceptance criteria," driving payload innovation from natural product modification to total synthesis.

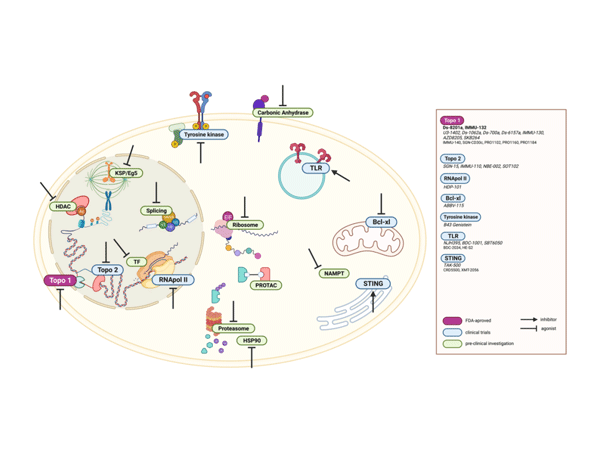
ADC Payloads Beyond Microtubule and DNA Alkylating Agents Schematic
二,Three-Generation Evolution: From "Chemotherapy Derivatives" to "Mechanism Innovators"
1.First Generation: "Cross-Border Trials" of Chemotherapy Drugs
Early ADCs directly borrowed classic chemotherapy drugs like methotrexate and doxorubicin, achieving targeted delivery but with significant defects such as insufficient toxicity and strong drug resistance. For example, doxorubicin-based ADCs had limited clinical effects due to poor payload water solubility and weak bystander effects, prompting a shift towards more efficient molecules.
2.Second Generation: "Precision Strangulation" by Microtubule Inhibitors
Represented by maytansinoids and auristatins, these payloads block microtubule polymerization or stabilize microtubule structure, arresting cancer cells in the G2/M phase:
- Maytansine Derivatives DM1/DM4: Derived from African shrubs, modified with a C3 side chain to obtain coupling sites, becoming the core payload of trastuzumab ADC (T-DM1) and pioneering a new paradigm in HER2-positive breast cancer treatment.
- Auristatins MMAE/MMAF: Synthetic analogs of sea hare toxins, with a tetrapeptide structure conferring high membrane permeability and significantly enhanced bystander effects for solid tumor killing, supporting the clinical success of ADCs like pertuzumab.
These payloads have IC50 values as low as nanomolar (nM), but their selectivity for mitotic cells limits their effect on dormant tumor cells.
3.Third Generation: "Genomic Detonation" by DNA-Damaging Agents
Shifting to DNA-targeting payloads that induce irreversible damage through mechanisms like double-strand breaks and alkylation:
- Enediyne Calicheamicin: One of the most potent natural cytotoxins, inducing DNA double-strand breaks 100 times more efficiently than chemotherapy drugs, supporting Mylotarg in treating acute myeloid leukemia.
- Topoisomerase I Inhibitor DXd: A camptothecin derivative overcoming the poor water solubility of traditional drugs, demonstrating bystander effect advantages in T-DXd with an objective response rate of 60% in HER2-low breast cancer.
- PBD Dimers: Selectively alkylating the minor groove of DNA, inducing apoptosis as a single agent, becoming a candidate for the next generation of high-specificity payloads.
These payloads act on the entire cell cycle and remain effective against low-antigen-expressing tumors, breaking through the target limitations of the second generation.
三,Diversified Innovation: From "Single Killing" to "Mechanism Crossover"
1.Targeting RNA: "Silent Killers" Sniper Dorman Cancer Cells
Payloads targeting RNA can attack both mitotic and dormant cells:
- Telanestin: Inhibits mRNA spliceosomes, blocking cancer cell gene expression. Pfizer has conjugated it with trastuzumab, showing efficient clearance of drug-resistant cell lines in vitro.
- Amanitin: Inhibits RNA polymerase II, with a water-soluble cyclic octapeptide structure conferring stability. HDP-101 targets BCMA for multiple myeloma treatment, with clinical trials showing deep remission potential.
2.Immune Activation: "Dual Weapons" Rewriting the Tumor Microenvironment
Immune ADCs (ISACs) move beyond direct killing modes to achieve "double strikes" by activating the immune system:
- TLR Agonists: Such as TLR7/8 agonists conjugated with anti-Her2 antibodies, driving dendritic cell maturation and CD8+ T cell recruitment, transforming "cold tumors" into "hot tumors," with companies like Heng Rui and BeiGene already deploying relevant pipelines.
- STING Agonists: Cyclodinucleotides (CDNs) conjugated with antibodies for targeted delivery, activating the type I interferon pathway. Mersana's XMT-2056 has received FDA orphan drug designation, with preclinical data for gastric cancer showing significant remodeling of the tumor microenvironment.
3.Novel Potential: "Future Stars" Breaking Traditional Frameworks
- Bcl-xL Inhibitors: Blocking anti-apoptotic proteins to induce programmed cancer cell death. AbbVie's ABBV-155 targets EGFR, reducing platelet toxicity, and showing inhibitory potential against solid tumors in clinical studies.
- PROTAC Molecules: Utilizing the proteasome degradation mechanism to introduce new "protein degradation" functions into ADCs, addressing traditional payload drug resistance.
- Near-Infrared Photoimmunotherapy: Photosensitizers like IR700DX as payloads, with light triggering tumor vascular destruction, pioneering a new field of "light-controlled precision killing."
四,Future Directions: From "Single Warhead" to "Smart Arsenal"
1.Dual-Payload ADCs: Simultaneously carrying microtubule inhibitors and DNA-damaging agents to synergistically attack multiple pathways and reduce drug resistance risks.
2.Personalized Customization: Designing payloads based on tumor genomic characteristics, such as PARP inhibitor payloads targeting BRCA-mutated tumors.
3.Multidisciplinary Integration: Combining AI drug design to optimize payload structure and utilizing gene editing technology to develop tumor-specific target conjugates.
4.Precision Toxicity Regulation: Balancing efficacy and safety through cleavable linkers and pH-responsive release, such as improving the normal tissue toxicity of calicheamicin.
Payloads – The "Engine Core" of ADC Transformation
From natural product extraction to total synthesis innovation, from cytotoxic molecules to immunomodulators, the evolution of payloads is the "combat effectiveness upgrade history" of ADC drugs. As the "cell division terminators" targeting microtubules, the "genome detonators" targeting DNA, and the "microenvironment remodelers" activating immunity synergize, ADCs are moving from "precision strikes" to "system collapse." In the future, with the implementation of new technologies like dual payloads and PROTAC, payloads will become the core hub connecting targeted therapy, immunotherapy, and gene therapy, propelling cancer treatment into a new era of "personalized precision guidance." Each payload innovation is a new "warhead" launched towards the goal of "curing cancer," and this continuously upgrading "weapons revolution" will ultimately rewrite the future landscape of cancer treatment.




