A New Era in Cancer Treatment: How ADC Drugs Rewrite Anti-Cancer Rules with "Precision Guidance + Super Warheads"

In the "arsenal" of cancer treatments, chemotherapy drugs have always been the mainstay. Like precision snipers, some block nucleotide synthesis, others disrupt DNA replication, and still others directly dismantle microtubules—the "steelworkers" that support the cell cytoskeleton. However, traditional chemotherapy is akin to carpet bombing, indiscriminately affecting both enemy and ally. It was not until the emergence of antibody-drug conjugates (ADCs) that "precision strikes" became possible.
- The Evolution of ADCs: From the "Bronze Age" to "King's Return"
The earliest attempts at ADCs date back to the 1950s, when scientists sought to "marry" murine antibodies with chemotherapy drugs. However, these first-generation ADCs were like "bronze warriors" with rudimentary equipment: poor targeting, low drug activity, and unfulfilled efficacy expectations despite unchanged toxicity. It was only in recent years, with breakthroughs in antibody engineering and linker chemistry, that ADCs truly achieved a "shotgun upgrade."
The ADCs approved today can be considered "super weapons," carrying "warheads" (payloads) with toxicities 1-2 orders of magnitude higher than traditional chemotherapy drugs. These "warheads" include:
- DNA Destroyers: Such as calicheamicin (DNA alkylating agent), SN-38 (topoisomerase inhibitor)
- Microtubule Terminators: Such as MMAE (microtubule inhibitor)
- Transcription Snipers: Such as DM1 (RNA polymerase inhibitor)
Payload Characteristics
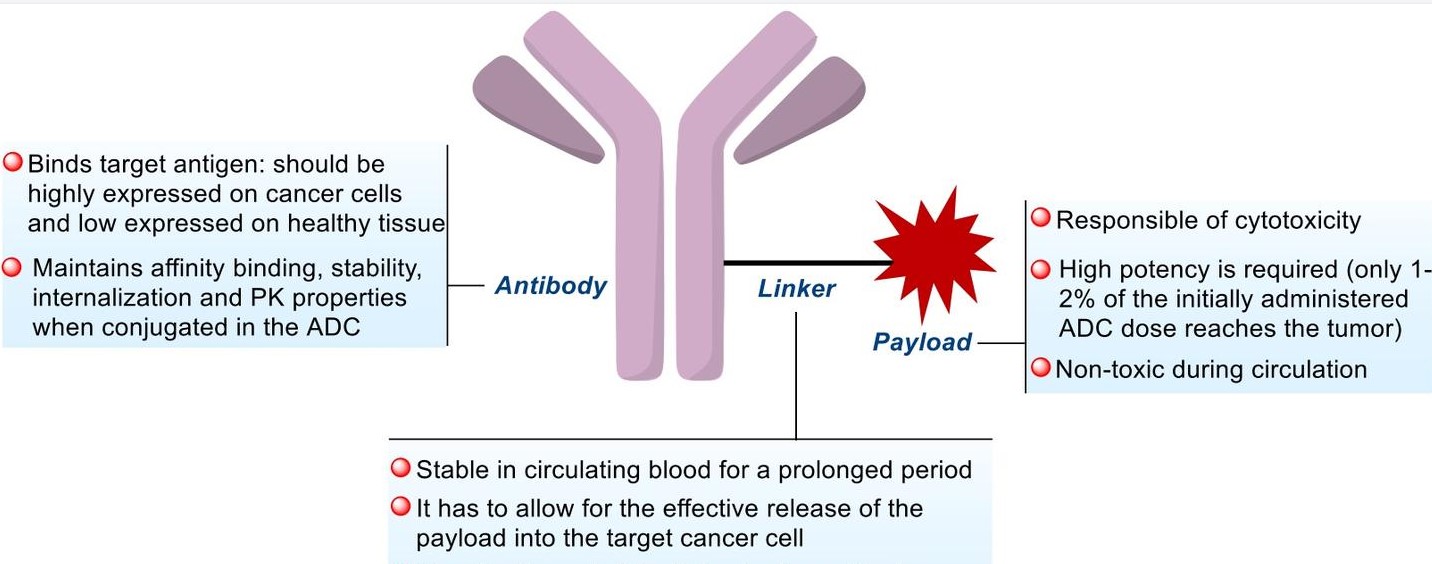
Payloads exert the intracellular cytotoxic activity of ADCs. The properties of cytotoxic agents covalently bound to antibodies via the linker moiety are crucial, as their mechanism of action will determine the efficacy of the resulting ADC as an anticancer compound and its potential indications. Ideal payloads possess the following characteristics: sufficiently high cytotoxicity; sufficiently low immunogenicity; high stability; modifiable functional groups without significantly affecting their potency; bystander killing effect; appropriate water solubility; and intracellular targets, as most ADCs need to enter tumor cells to release their payloads.
(Data source: Cheng-Sánchez I, et al. Mar Drugs. 2022)
- The Arrival of New "Warheads": Five Killer Moves Beyond Tradition
As the limitations of existing "warheads" became apparent, scientists began to explore new, more lethal payloads:
-
Ducamycin: DNA's "Liquefaction Weapon"
As a DNA alkylating agent, ducamycin can penetrate cell membranes, even affecting multidrug-resistant cells with "impenetrable walls." The ADC SYD985 acts like a "smart missile," precisely cleaved by cathepsin B to release toxic cyclopropane that attacks DNA. -
PNU-159682: The "Ultimate Evolution" of Anthracyclines
Not cytotoxic enough? PNU-159682, a liver metabolite of doxorubicin, is 100 times more toxic, specifically targeting DNA topoisomerase II and bypassing the "defense system" of efflux transporters. -
Amanitin: The "Terminator" of RNA Synthesis
This bicyclic peptide extracted from poisonous mushrooms can efficiently inhibit RNA polymerase II, leaving even dormant tumor stem cells nowhere to hide. HDP-101 transforms it into an "immunoactivating warhead," demonstrating synergistic effects when combined with immune checkpoint inhibitors. -
Tubulysins: "Disassembly Experts" of Microtubules
These tetrapeptides extracted from myxobacteria are 1000 times more toxic than vinblastine. MEDI4276 employs a bispecific antibody design, gripping cancer cells like "double-sided tape" and releasing tubulysin derivatives to block microtubule polymerization. -
Eribulin: The "Freezing Device" of Microtubules
As a synthetic analog of sponge extracts, eribulin can freeze microtubule dynamics and remains effective against paclitaxel-resistant cells. MORAb-202 combines it with folate receptor antibodies, demonstrating best-in-class potential in clinical trials.
- Preclinical Research: Installing "Safety Valves" for "Super Weapons"
To make these "warheads" both efficient and safe, they must undergo rigorous testing:
- Plasma Protein Binding: Tracking binding rates at low concentrations with radiolabeled tracers
- Tissue Distribution: Precisely locating through quantitative whole-body autoradiography (QWBA)
- Metabolic Studies: Ensuring rapid metabolism of "warheads" in the circulatory system to reduce systemic toxicity
- Transporter Influence: Avoiding the "defense network" of efflux transporters to ensure effective accumulation in tumor tissues
- Future Battleground: ADC's "Cross-Border Expedition"
The ideal ADC "warhead" should meet the following criteria:
- Unique Mechanism of Action: Avoiding cross-resistance
- pM-Level Lethality: Ensuring efficient killing
- Bystander Effect: Eliminating heterogeneous tumor cells
- Rapid Clearance: Reducing systemic toxicity
With the emergence of innovations such as dual warhead designs, radionuclide payloads, and PROTAC technology, the indications for ADCs are expanding from oncology to areas like infections and autoimmune diseases. This revolution in cancer treatment, led by "precision guidance + super warheads," is rewriting the rules of human cancer fighting.




