Introduction to the popular application of TR-FRET - drug discovery and development
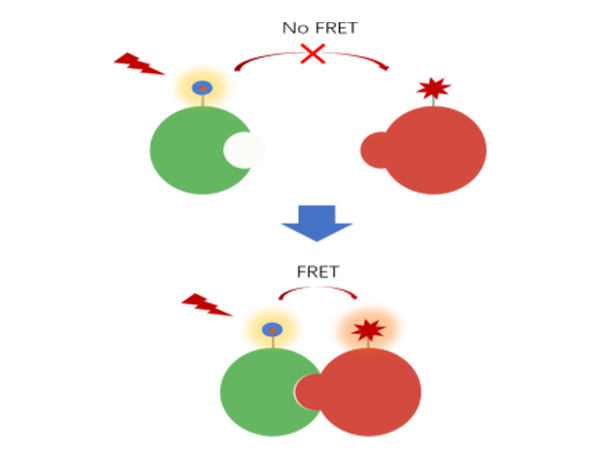
TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer) is a biophysical technique used to study intermolecular interactions. It combines two techniques: fluorescence resonance energy transfer (FRET) and time-resolved fluorescence (TRF).
Fluorescence resonance energy transfer is a physical phenomenon in which a fluorescent molecule (donor), after absorbing light energy, can transfer excitation energy non-radioactively to another molecule (acceptor) if the acceptor is spatially close enough to the donor (usually in the range of 1-10 nm). The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between the donor and acceptor, so it can be used to study the proximity and interactions between molecules.
Time-resolved fluorescence (TRF) is a technique for measuring fluorescence lifetime that distinguishes between short-lived background fluorescence and long-lived signal fluorescence by measuring fluorescence decay after the excitation of the light source has been stopped. This allows fluorescence to be measured within a specific time window after excitation, reducing background interference and improving the signal-to-noise ratio of the signal. Using this technology, it can efficiently screen targeted protein/protein receptor inhibitors, screen PROTAC molecules for targeted protein degradation, and screen KRAS/cRAF small molecule blockers.

Schematic diagram of fluorescence resonance energy transfer

Schematic diagram of time-resolved fluorescence
1. Cytokine/cytokine receptor blocker screening
Cytokine inhibitors have shown a wide range of potential in the medical community in terms of application and research, especially in the treatment of autoimmune diseases, tumors, infections, and many other diseases.
In the treatment of autoimmune diseases, IL-2 and IFN are important cytokine inhibitors for the treatment of autoimmune diseases. IL-2 has been extensively studied for the treatment of a variety of autoimmune diseases and, despite challenging development, remains one of the key drugs for the treatment of these diseases. IFN-α is mainly used for the treatment of viral infections and tumors, and its efficacy in chronic active hepatitis and hairy cell leukemia is significant. Recently, inhibitors targeting B lymphocyte stimulating factor (BLyS), such as belimumab, have been approved in China, and more than 20 drugs are in active development for the treatment of other autoimmune diseases such as rheumatoid arthritis. IL-1 receptor antagonists have shown good efficacy in the treatment of inflammatory and autoimmune diseases.
In the direction of tumor treatment, inhibition of specific cytokine signaling pathways in tumor cells is one of the current strategies for tumor treatment. For example, the application of anti-TNF monoclonal antibodies in the treatment of tumors, as well as novel therapies for the treatment of tumors using PD-L1/IL-15 immunocytokines, are being developed. Small-molecule protein-protein interaction inhibitors, which inhibit intracellular signaling, are used in cancer therapy, particularly by blocking tumor signaling pathways by inhibiting key signaling molecules such as STAT3. In addition, CDK4/6 inhibitors have shown potential in the treatment of breast cancer, and three drugs have been approved for the treatment of patients with hormone receptor-positive breast cancer.
In the treatment of infectious and inflammatory diseases, cytokine production and receptor binding that inhibit inflammatory response are effective methods for the treatment of septic shock and autoimmune diseases.
Cytokine inhibitors have shown significant efficacy in the treatment of many diseases, but they also face challenges such as high side effects and drug resistance. With the development of new technologies and new drugs, there may be more options and better efficacy in the future.

Fig.1 TR-FRET technology was used to screen cytokine blockers
As shown in the figure, the interaction between Tag1 and Tag2 was detected by using an Eu-tagged anti-Tag1 antibody (TR-FRET donor) and an Accepter-tagged anti-Tag2 antibody (TR-FRET receptor). Since the binding of Tag1 to Tag2 mediates the proximity of the donor antibody to the recipient antibody, the excitation of the donor antibody triggers a fluorescence resonance energy transfer (FRET) to the recipient antibody, causing the recipient antibody to specifically emit a signal at a wavelength of 665 nm. This particular signal is directly proportional to the degree to which Tag1 interacts with Tag2. This homogeneous experiment is simple to perform and does not require a washing step.
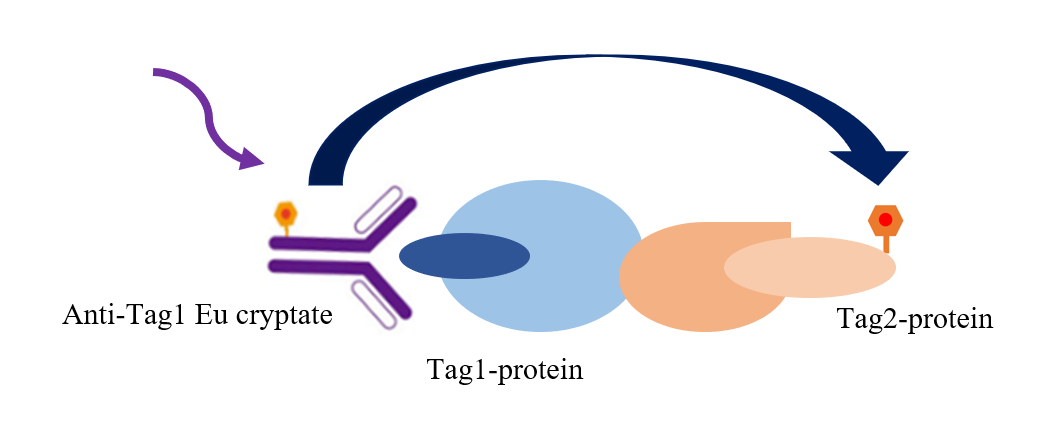
Fig.2 TR-FRET technology was used to screen cytokine blockers
As shown in the figure, the interaction between Tag1 and Tag2 was detected by using an Eu-tagged anti-Tag1 antibody (TR-FRET donor) and an Accepter-tagged Tag2 protein (TR-FRET receptor). Since the binding of Tag1 to Tag2 mediates the proximity of the donor antibody to the recipient antibody, the excitation of the donor antibody triggers a fluorescence resonance energy transfer (FRET) to the recipient antibody, causing the recipient antibody to specifically emit a signal at a wavelength of 665 nm. This particular signal is directly proportional to the degree to which Tag1 interacts with Tag2. This homogeneous experiment is simple to perform and does not require a washing step.
Related product recommendations
|
Catalog number |
Product name |
specification |
|
abs560014 |
Human TNF-α/TNFR1 Binding Kit |
500T |
|
abs560015 |
Human TNF-α/TNFR2 Binding Kit |
500T |
|
abs560017 |
Human IL2/IL2R Binding Kit |
500T |
|
abs560018 |
Human IL3/IL3R Binding Kit |
500T |
|
abs560019 |
Human IL4/IL4R Binding Kit |
500T |
|
abs560020 |
Human IL6/IL6R Binding Kit |
500T |
|
abs560021 |
Human IL17A/IL17RA Binding Kit |
500T |
Data Display: The following data is not a substitute for the data obtained in the experiment, and is intended as an example only, and results may vary depending on the TR-FRET compatible instrument.
Data Display: The following data is not a substitute for the data obtained in the experiment, and is intended as an example only, and results may vary depending on the TR-FRET compatible instrument.
1、TNF-a & TNFR1 Binding

2、TNF-a & TNFR2

3、IL-2 & IL-2R

4、IL-3 & IL-3R
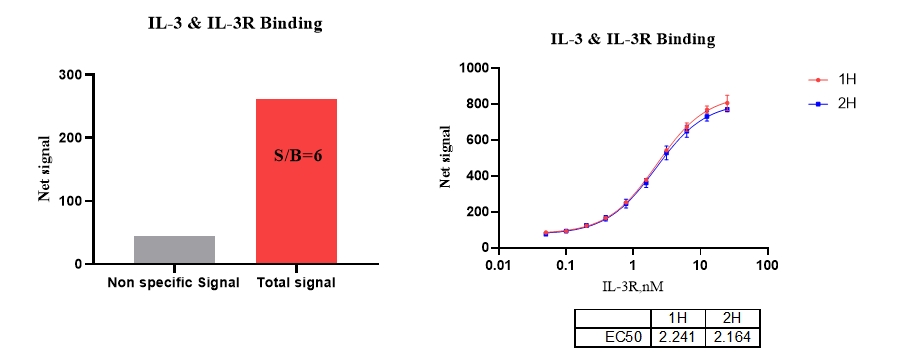
5、IL-4 & IL-4R

6、IL-17 & IL-17RA

2. PROTAC molecular screening system for targeted protein degradation
Proteolysis Targeting Chimeras (PROTACs) are an emerging drug design strategy that uses the intracellular ubiquitin-proteasome system to selectively degrade target proteins. In recent years, with the continuous development of PROTAC technology, its application has far exceeded the original scope of cancer treatment, and expanded to include immune diseases, neurodegenerative diseases, cardiovascular diseases, infectious diseases and other fields.
In the direction of cancer treatment, PROTAC technology has made significant progress in cancer treatment. For example, ARV-110 has entered Phase I clinical trials for the treatment of metastatic castration-resistant prostate cancer as an oral small molecule PROTAC targeting the androgen receptor (AR). In the study of non-small cell lung cancer, a PROTAC molecule with high potency has emerged and has shown good efficacy in vitro and in vivo experiments. In addition, PROTAC molecules targeting EGFR have also shown advantages in the treatment of non-small cell lung cancer. While traditional targeted therapies often lead to resistance due to mutations or overexpression of the target protein, PROTAC technology has shown the potential to overcome these resistance mechanisms by degrading rather than simply inhibiting the target protein. For example, PROTACs offer a new treatment option for drug resistance, which is common in cancer treatment.
PROTAC technology is not limited to cancer treatment, but also shows promising applications in a variety of other diseases, such as cardiovascular diseases, neurological diseases, metabolic diseases, etc. For example, ARV-471, an ER-targeted oral drug developed using PROTAC technology, has shown potential in clinical trials for the treatment of ER-positive, HER2-negative breast cancer. This technology allows for drug development for targets that are traditionally considered "undruggable", providing new avenues for drug discovery. For example, PROTACs offer a new solution to the problem of non-specificity and resistance to kinase inhibitors.
PROTAC technology has attracted a lot of attention from academia and industry, showing great potential in drug development. However, there are still many challenges, such as how to improve its stability and cell permeability inside and outside the cell, and how to overcome the potential side effects caused by this. In conclusion, PROTAC technology provides new possibilities for the treatment of a variety of diseases through its unique mechanism of action, especially in overcoming the limitations of traditional drugs.
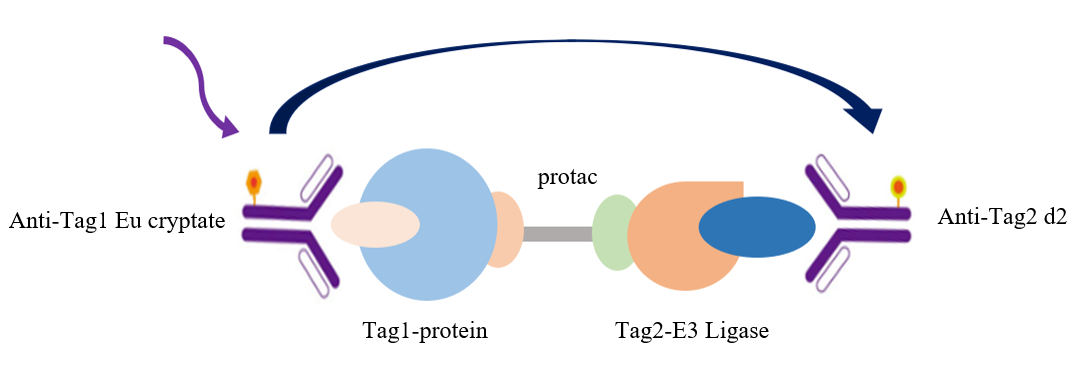
Fig.3 TR-FRET technology was used to screen the principle model of PROTAC molecular technology
As indicated, the interaction between Tag1 and Tag2 is detected by using an Eu-labeled anti-Tag1 antibody (TR-FRET donor) and an Accepter-labeled anti-Tag2 antibody (TR-FRET receptor). Since the molecular glue PROTAC mediates the interaction between Tag1 and Tag2, the donor antibody is close to the recipient antibody, and the excitation of the donor antibody triggers the fluorescence resonance energy transfer (FRET) to the recipient antibody, so that the recipient antibody specifically emits a signal with a wavelength of 665 nm. This particular signal is directly proportional to the degree of interaction between PROTAC and Tag1/Tag2. This homogeneous experiment is simple to perform and does not require a washing step.
Related product recommendations
|
Catalog number |
Product name |
specification |
|
abs560016 |
Human BRD4/CRBN PROTAC Binding Kit |
500T |
|
abs560012 |
Human DDB1-CRBN&GSPT1 Binding Kit |
500T |
Data Display: The following data is not a substitute for the data obtained in the experiment, and is intended as an example only, and results may vary depending on the TR-FRET compatible instrument.
1、DDB1/CRBN & BDR4,Protac : BET Degrader-1

2、DDB1/CRBN & GSPT1,Protac : CC-885

3. KRAS/cRAF small molecule blocker screening system
KRAS protein is a GTPase whose function is to hydrolyze GTP into GDP, which is an important step in the process of intracellular signaling. The KRAS protein is a member of the RAS family and is one of the most commonly mutated genes in tumorigenesis, accounting for approximately 20-25% of human tumors. It has mutations in a variety of cancer types, including lung, colorectal, and pancreatic cancers. Mutations in the KRAS protein often lead to its persistent activation, which triggers multiple cancer signaling pathways that promote cancer cell proliferation, survival, and metastasis.
There are different isoforms of mutations in the KRAS protein, including G12C, G12D, and G12V, among others, with the G12C mutation being the most common. Small molecule inhibitors targeting the KRAS G12C mutation have entered clinical trials and have shown potential therapeutic efficacy in patients with non-small cell lung cancer and colorectal cancer.
MRTX1133 is a small molecule inhibitor developed by Mirati Therapeutics that targets the KRAS G12D mutant, the most common form of KRAS mutation, which is widely present in a variety of solid tumors, especially in pancreatic ductal adenocarcinoma (PDAC), where KRAS mutations are present in approximately 93% of cases, with G12D and G12V being the most prevalent. MRTX1133 is the first non-covalent, potent and selective inhibitor of KRAS G12D, with good clinical performance.
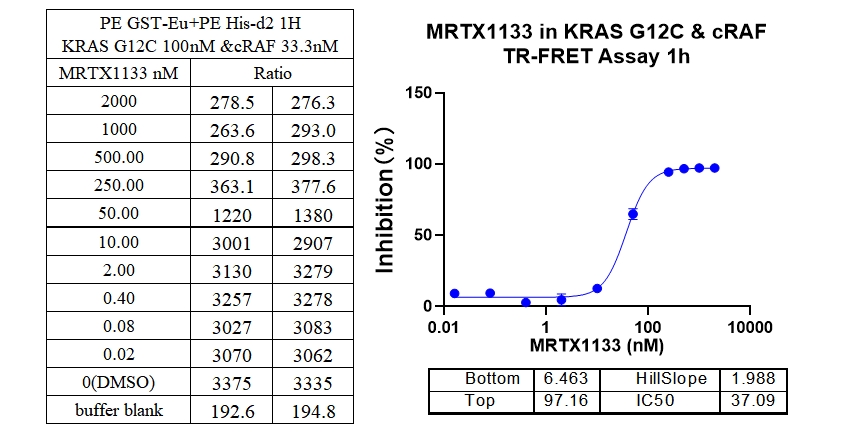
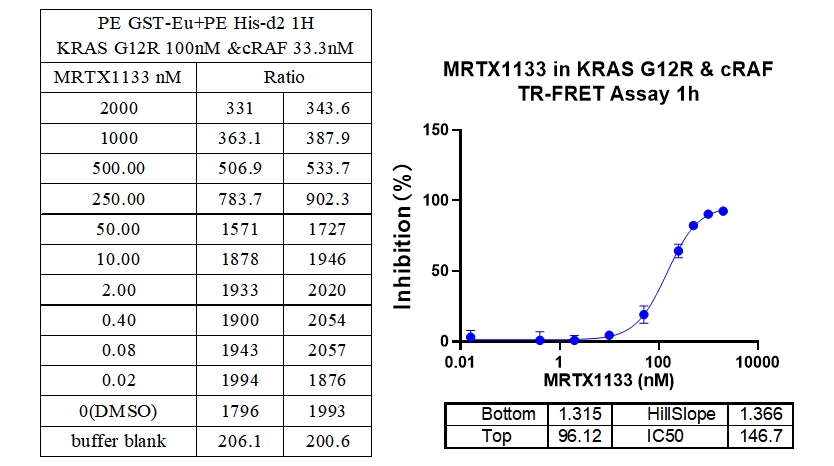
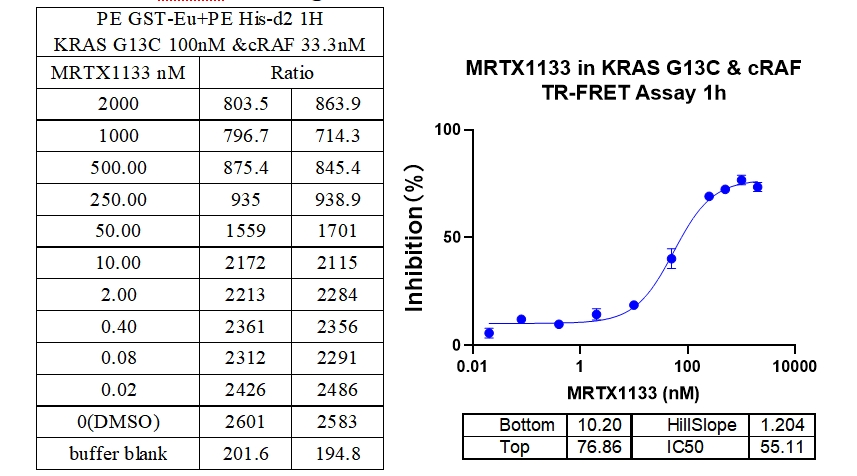
In this experiment, Europium-labeled anti-6xHis and Acceptor-labeled GST were used to detect the interaction between different mutations of Kras protein and cRAF (RAF1) protein, MRTX1133 a 2-fold serial dilution from 300 nM, and the signal was detected after 1 h of incubation. The data showed a good S/B window (~7x), high sensitivity and selectivity (G12D IC50 was the smallest).

Fig.4 TR-FRET technology is used to screen the technical principle model of KRAS/cRAF small molecule blocker
As shown, the KRAS WT/GTP/cRAF interaction was detected by using an Eu-labeled anti-Tag1 antibody (TR-FRET donor) and an accepter-labeled anti-Tag2 antibody (TR-FRET receptor). Due to the KRAS WT/GTP/cRAF interaction, the donor antibody is in close proximity to the recipient antibody, and the excitation of the donor antibody triggers fluorescence resonance energy transfer (FRET) to the recipient antibody, causing the recipient antibody to specifically emit a signal at a wavelength of 665 nm. This particular signal is directly proportional to the degree of interaction of KRAS WT/GTP/cRAF. This homogeneous experiment is simple to perform and does not require a washing step.
Product kits have been launched
|
Catalog number |
Product name |
specification |
|
abs560003 |
Human KRAS WT&cRAF Binding Kit |
500T |
|
abs560004 |
Human KRAS G12C&cRAF Binding Kit |
500T |
|
abs560005 |
Human KRAS G12D&cRAF Binding Kit |
500T |
|
abs560006 |
Human KRAS G12R&cRAF Binding Kit |
500T |
|
abs560007 |
Human KRAS G12V&cRAF Binding Kit |
500T |
|
abs560008 |
Human KRAS G13C&cRAF Binding Kit |
500T |
|
abs560009 |
Human KRAS G13D&cRAF Binding Kit |
500T |
|
abs560010 |
Human KRAS Q61H&cRAF Binding Kit |
500T |
Data Display: The following data is not a substitute for the data obtained in the experiment, and is intended as an example only, and results may vary depending on the TR-FRET compatible instrument.
1、KRAS WT & cRAF Binding, Inhibitor : MRTX1133

2、KRAS G12C & cRAF Binding, Inhibitor : MRTX1133
3、KRAS G12D & cRAF Binding, Inhibitor : MRTX1133

4、KRAS G12V & cRAF Binding, Inhibitor : MRTX1133

5、KRAS G12R & cRAF Binding, Inhibitor : MRTX1133
6、KRAS G13C & cRAF Binding, Inhibitor : MRTX1133
7、KRAS G13D & cRAF Binding, Inhibitor : MRTX1133

8、KRAS Q61H & cRAF Binding, Inhibitor : MRTX1133

References:
[1] Small Molecule Protein–Protein Interaction Inhibitors as CNS Therapeutic Agents: Current Progress and Future Hurdles.Richard R Neubig; Levi L Blazer
[2] Effects of 17-DMAG on non-small cell lung cancer cell lines A549 and H1975 being resistant to EGFR-TKI.Zhao, Lei; Cao, Fumin
[3] Latest Overview of the Cyclin-Dependent Kinases 4/6 Inhibitors in Breast Cancer: The Past, the Present and the Future.Zhang, Wei; Chen, Xiu; Xu, Weilin ; et al.
[4] Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: Recent progress and future challenges,Zeng, Shenxin; Huang, Wenhai; Liyan Cheng; et al.
[5] Overcoming Cancer Drug Resistance Utilizing PROTAC Technology.Zheng, Guangrong; Burke, Matthew R; Smith, Alexis R




