DC cell culture strategy
Overview
In 1973, Steinmen and Cohn of Rockeller University in the United States first discovered a cell with many dendritic protrusions on the surface from the isolation of mouse splenocytes, and named it dendritic cells (DCs). As the commander of the immune front, DC cells are the most powerful antigen-presenting cells in the body known, and a single DC cell can activate 100-3000 T cells, which is 100-1000 times more than macrophages and B cells[1]。 DC cells initiate anti-tumor immunity by processing tumor antigens and presenting them to T cells, linking innate immunity to adaptive immunity, so DC cells are the initiators of the body's immune response and have a unique position in the induction of immune responses.
1. DC cell source
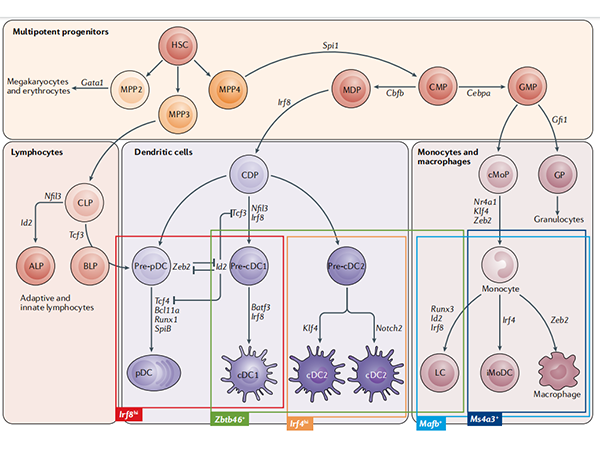
DC cells are lymphoid or myeloid cells that originate in the bone marrow and inhabit peripheral and lymphoid tissues. Most DCs are generated by common DC progenitor cells (CDPs), which are eventually induced by FLT3L to form cDCs and pDCs. A portion of monocytes derived from hematopoietic stem cells will produce peripheral blood mononuclear cell-derived DCs induced by GM-CSF and IL-4[2]。
So the source of DC cells cultured in vitro can be:Monocyte induction in bone marrow and peripheral blood; or induction of CD34+ HSC bone marrow pluripotent hematopoietic stem cells derived from bone marrow and peripheral blood; or directly isolated from samples such as blood, tissue, etc. Among them, CD14+ monocytes in peripheral blood are more convenient to obtain, and induction of Mo-DC cells is the most classic method, but Mo-DCs cannot be recycled to lymph nodes, and there is a difference between Mo-DCs and lymphoid tissue-resident dendritic cells (LT-DCs).[3]; CD34+ HSC cells are not readily available but can induce the production of pDCs, cDC1 and cDC2. DC cells isolated directly from the sample are closest to the real situation in vivo, but the yield is generally low. Another novel source of DC cells is that induced pluripotent stem cells iPSCs/embryonic stem cells ESCs can also be induced into DC cells: after iPSCs are differentiated into CD34+ hematopoietic stem cells, they can be differentiated into dendritic cells (iDCs) by adding GM-CSF, IL4, TNF-α, OK432 culture systems, and iDCs can be directly genetically edited on iPSCs to introduce specific antigen genes. This greatly shortens the time it takes for DCs to incubate antigens and present them to T cells[4]。 Of course, you can also directly purchase commercial DC cells, which is convenient, fast and has guaranteed quality.
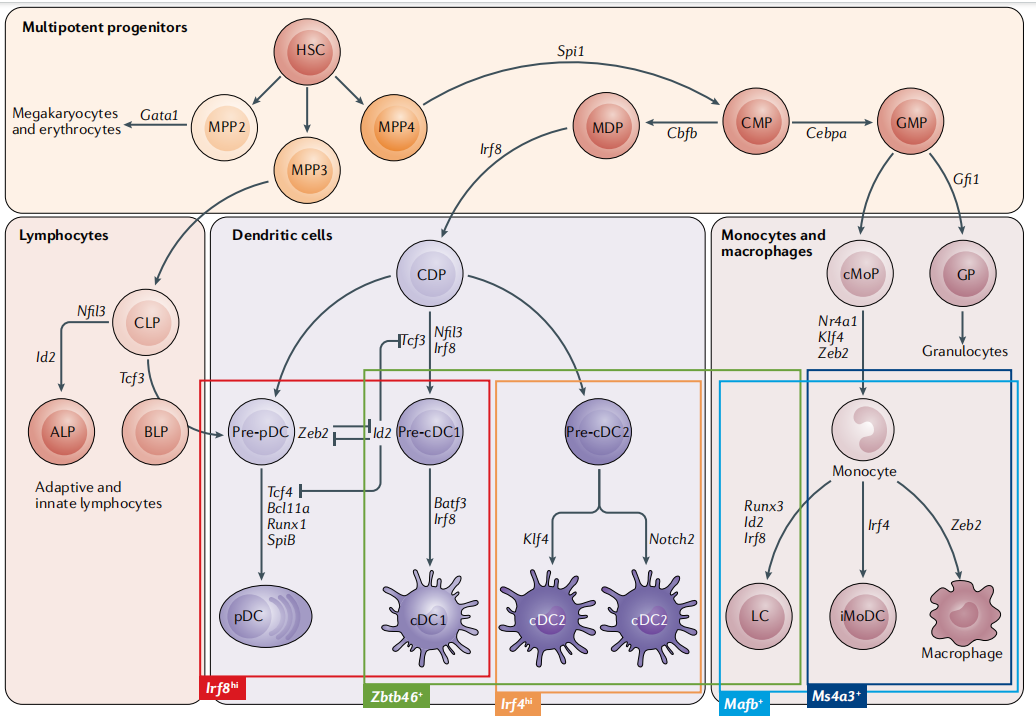
Fig.1 Map of mouse dendritic cell development[2]
2. DC-induced differentiation and culture
Since the sample sources and induction methods of DC cells are also relatively complex, here are three examples:
1) Human bone marrow CD34+ cells induce DCs[5]
(1) Culture and inoculation of feeder cells OP9
a. OP9 cells at 75 cm2Incubate in flasks to a density of 4×103-1×104Cells/cm2, OP9 medium (αMEM, 20% FBS, penicillin-streptomycin), 37 °C, 5% CO2;
b. Harvest OP9 cells, add 2-3mL of trypsin-EDTA solution to the culture flask, digest at 37 °C for 5-15min, and observe the cell layer under an inverted microscope until the cells become round and begin to fall off. Add 6-8 mL of OP9 complete medium, terminate digestion, and gently pipette the cells;
c. Transfer the cell suspension to a 15mL centrifuge tube, centrifuge at 125×g for 10min, discard the supernatant, and resuspend the OP9 cell pellet in fresh pre-warmed medium with a cell density of 2.5×104cells/mL;
d. Aliquot 200 μL (5000 cells) per well into a 96-well U-bottom tissue culture treatment plate at 37 °C with 5% CO2Medium culture.
(2) DC differentiation
a. Aspirate the medium of pre-seeded OP9 cells and replace it with 200 μL of CD34+ cell suspension (3000 cells) to continue the culture;
On day 6, 100 μL of medium was removed from e ach well and 100 μL of pre-warmed DC differentiation medium αMEM (abs9506), 10% FBS (abs972), 1% penicillin-streptomycin (abs9244), 100ng/mL Flt3L (abs04375), 20ng/mL SCF (abs04178), 20mg/mL GM-CSF (abs00839); Cells are harvested on days 12-21 and can be fed half-volumes on days 12 and 18. Human bone marrow CD34+ cells induce more DC cell subtypes, as shown in Figure 2.
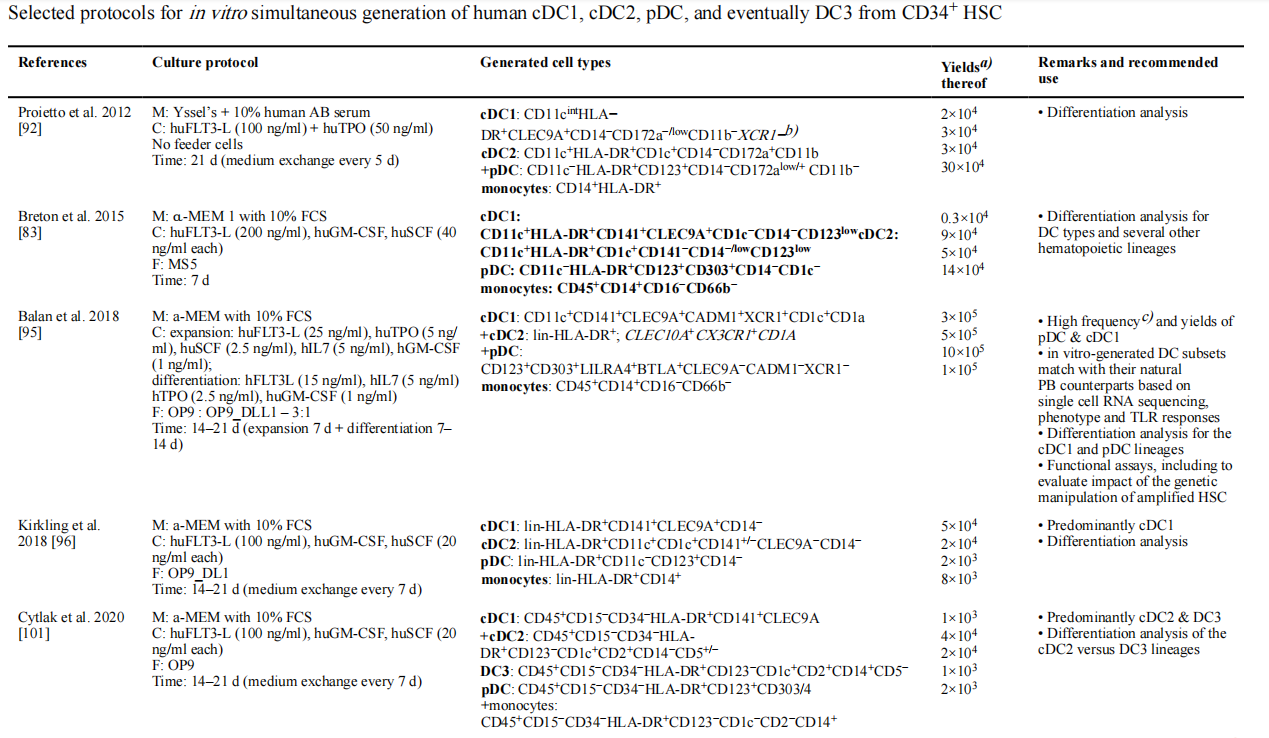
Fig.2 Summary of human bone marrow CD34+ cells induced to generate more DC cell subtypes[5]
Some of the related products in this article:
|
Catalog number |
Product name |
specification |
|
abs9506 |
α-MEM medium (with nucleosides, with deoxynucleosides, with L-glutamine, with sodium pyruvate, without HEPES) |
500mL |
|
abs972 |
Fetal bovine serum (Premium) |
500mL |
|
Penicillin-streptomycin solution (100 ×, bispecific antibody) |
100mL |
|
|
abs47048304 |
Trypsin-EDTA digest (0.05% with phenol red) |
100mL |
|
abs47048305 |
Trypsin-EDTA digestion solution (0.05%, phenol red free) |
100mL |
|
abs04375 |
Recombinant Human FLT3LG Protein(C-6His) |
50μg |
|
abs04178 |
Recombinant Human SCF Protein |
50μg |
|
abs00839 |
Recombinant Human GM-CSF Protein |
5μg |
2) Monocytes induce MoDCs[6]
(1) Adjust the concentration of enriched monocytes to 5×10 with medium RPMI 1640 (containing 10% FBS).6cells/mL, plated on 24-well plates, and recombinant human IL-4 (abs04698) (500 U/mL) and GM-CSF (abs00839) (500 U/mL) were added at 37 °C, 5% CO2Incubated in incubators;
(2) After 48 hours, half of the fluid was exchanged, and recombinant human IL-4 (500U/mL) and GM-CSF (500U/mL) were supplemented at the same time, and the stimulation was continued for 2 days;
(3) On the 5th day, immature dendritic cells, i.e., monocyte-derived dendritic cells (MoDCs), can be harvested for downstream experiments (such as activation of mature DCs);
(4) Mature DC activation: LPS (0.5 μg/mL) (abs47014848), pha (10 ng/mL) (abs47014910), IL-6 (10 ng/mL) (abs04044), IL-1β (10 ng/mL) (abs00802), PGE2 ( 1 μmol/L) (abs819421) stimulates for 24-48h.
Some of the related products in this article:
|
Catalog number |
Product name |
specification |
|
abs9484 |
RPMI 1640 medium (with L-glutamine, without HEPES) |
500mL |
|
abs04698 |
Recombinant Human IL-4 Protein |
100ug |
|
abs00839 |
Recombinant Human GM-CSF Protein |
5ug |
|
abs47014848 |
Lipopolysaccharide (O55:B5) |
10mg |
|
abs47014910 |
Phytohemagglutinin-L solution (500×) |
100uL |
|
abs04044 |
Recombinant Human IL-6 Protein |
50ug |
|
abs00802 |
Recombinant Human IL-1β Protein |
10ug |
|
abs819421 |
Prostaglandin E2 |
5mg |
3) IPSC/ESC induces DC cells[7]
(1) Differentiation of IPS cells (or ES cells) into hematopoietic progenitor cells
When IPS or ES cells reach 70-80% density, use Collagenase IV (abs47048003) at 37 °C, 5% CO2Digestion for 40-60min until the edge of the cell mass is rolled up and the digestion is terminated;
b. Cell mass formation: IPS or ES cell mass is filtered through the cell sieve to adjust the cell mass size to form a cell mass containing 50-100 cells;
c. If IPS or ES cell clusters are to be cultured on mouse embryonic fibroblasts (MEFs), the MEFs should be removed before inoculation; at 37°C, 5% CO2 and 5% O2Conditioned culture, the medium is changed daily to form embryoid body EBs;
From day 1, prepare the corresponding induction medium (D1, D2, D4, D6) according to the number of days of differentiation;
Hematopoietic progenitor cells were observed to emerge from EB during days 6 to 14 (IPS cells) or days 21 to 28 (ES cells) of differentiation.

Fig.3 D2-D6 EB differentiation stage medium
(2) IPS cells (or ES cells)-derived hematopoietic progenitor cells differentiate into DC subsets
Radiation-inactivated OP9 cells were treated at 1.2x104cells/cm2Density inoculated on Petri dishes coated with 0.1% gelatin; Hematopoietic progenitor cells are collected and filtered through a 40 μm cell sieve to remove residual EBs; Adjust the cell density to 1x106-1.5x106cells/mL in DC differentiation basal medium;
b. Divide the cells into two groups, one for the generation of cDC1 and cDC2 (using the FSG4 cytokine combination) and the other for the generation of cDC1 and pDCs (using the FSG cytokine combination);
100 ng/mL FLT3L (abs04375) and 20 ng/mL SCF (abs04178) were added to the FSG4 group , 20ng/mL GM-CSF (abs00839) and 20ng/mL IL-4 (abs04698);
100 ng/mL FLT3L (abs04375) and 20 ng/mL SCF (abs04178) were added to the FSG group and 10ng/mL GM-CSF (abs00839);
c. Inoculating the hematopoietic progenitor cell suspension onto OP9 stromal cells for co-culture; Perform a half-volume fluid exchange every two days; On the 4th to 7th day of differentiation, the DC morphology will gradually appear.
Some of the related products in this article:
|
Catalog number |
Product name |
specification |
|
abs47048003 |
Collagenase type IV |
100mg |
|
abs06016 |
Recombinant Human VEGF 121 Protein(C-10His) |
50ug |
|
abs05479 |
Recombinant Human IGF-I Protein |
50ug |
|
abs04218 |
Recombinant Human TPO Protein(N,C-6His) |
50ug |
|
abs05292 |
Recombinant Human IL-3 Protein |
50ug |
3. Identification of DC cell subtypes
DC cell subsets can be divided into plasma DC, type 1 myeloid DC, type 2 myeloid DC, etc., and different subtypes, markers, and cytokines of DC cells can be referred to Figure 4. The most commonly used method for the identification of DC cell isotypes is flow cytometry, and you can refer to the "Classification and Identification of Immune Cell Culture Strategy (III)" for the basic content of flow cytometry identification.
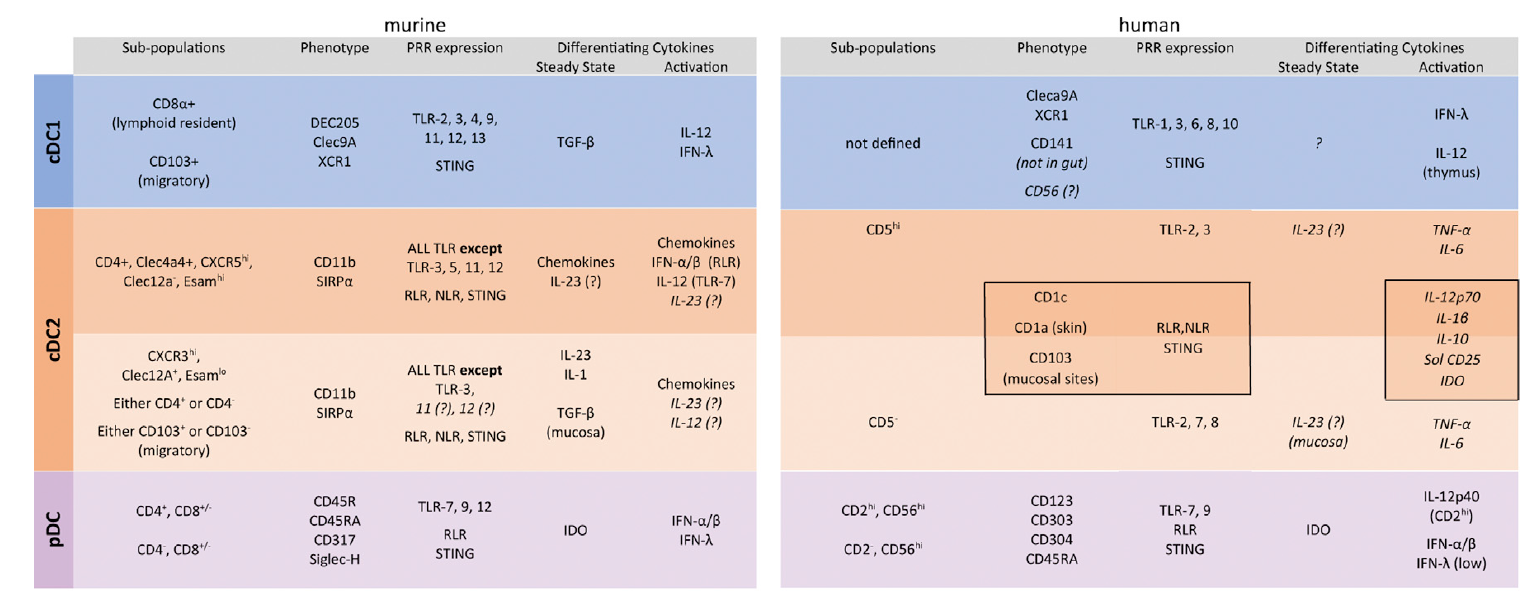
Fig.4 Surface markers, pattern recognition receptors (PRRs), and cytokines of mouse and human DC subsets[8]
4. Popular research on DC cells
When the function of DC cells is impaired, tumor cells are likely to use this aspect to produce immune escape, and the tumor microenvironment will also release many inhibitors, thereby preventing the normal differentiation of DC progenitor cells, thereby inhibiting the anti-tumor response of T cells[9]。
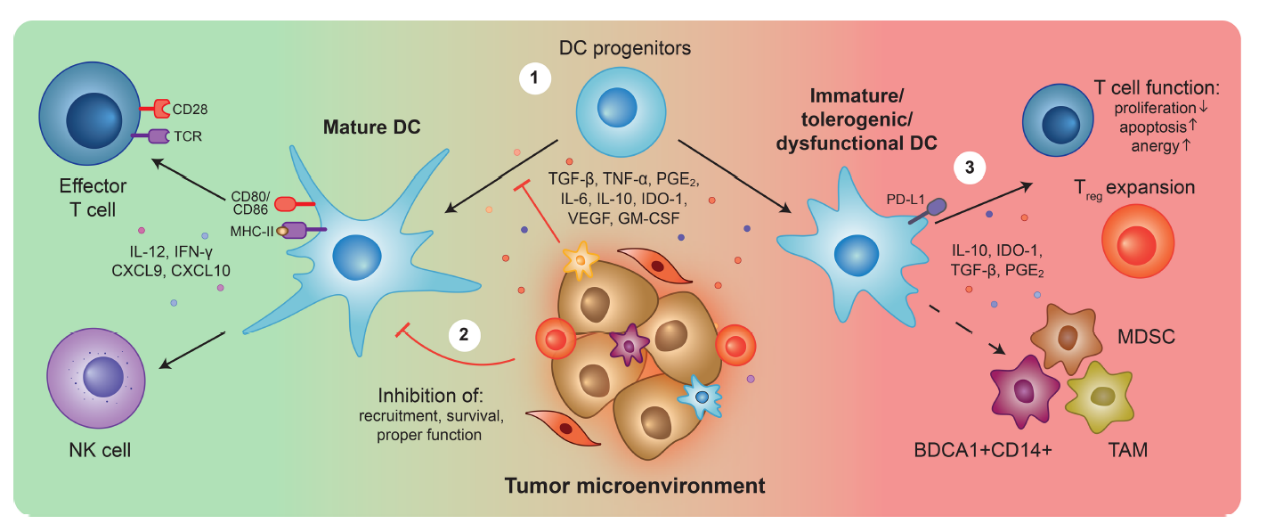
Fig.5 DC cells and tumor microenvironment[9]
There are many popular studies on DC cells: tumor-infiltrating DC cells, myeloid suppressor cells, DC vaccines, DC-CIK cell therapy, DC-T cell therapy, etc.[10]: CD11c+MHCII+ double-positive myeloid cells→CD317+(pDC)→ CD64+F4/80-(moDC→ CD103+CD11b−(cDC1), CD103-CD11b+(cDC2). In addition to flow cytometry, there are many powerful tools for studying tumor-infiltrating DC cells: mass spectrometry, single-cell sequencing, and more (Figure 7).
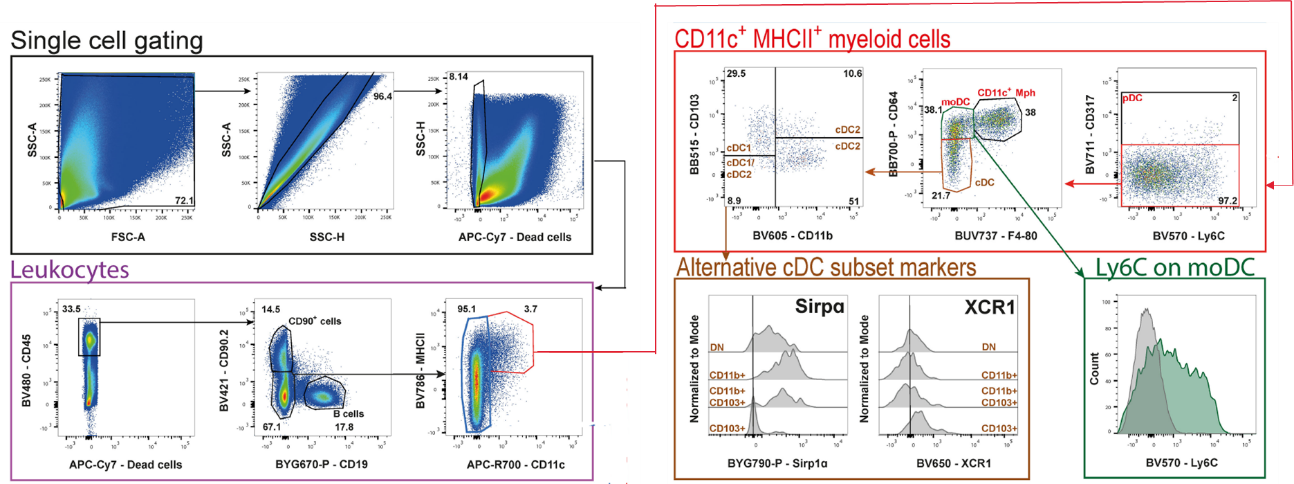
Fig.6 DC flow cytometry analysis of mouse colon cancer tumor infiltrating DC subtype gating logic[10]
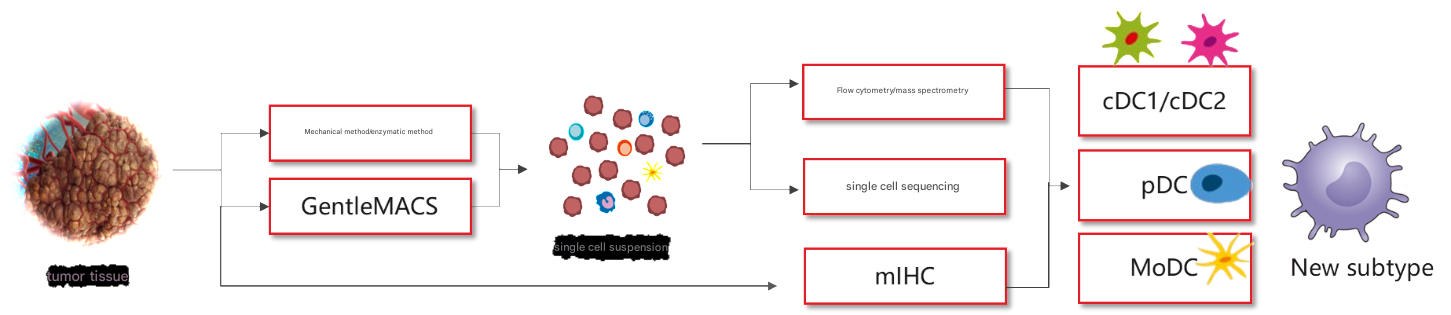
Fig.7 Summary of research ideas on tumor-infiltrated DC cells
References:
[1] Hart, D.N.J., Dendritic Cells: Unique Leukocyte Populations Which Control the Primary Immune Response. Blood, 1997. 90(9): p. 3245-3287.
[2] Anderson, D.A., et al., Genetic models of human and mouse dendritic cell development and function. Nature Reviews Immunology, 2020. 21(2): p. 101-115.
[3] Balan, S., et al., Large-Scale Human Dendritic Cell Differentiation Revealing Notch-Dependent Lineage Bifurcation and Heterogeneity. Cell Reports, 2018. 24(7): p. 1902-1915.e6.
[4] Xue, D., et al., Induced pluripotent stem cell-derived engineered T cells, natural killer cells, macrophages, and dendritic cells in immunotherapy. Trends in Biotechnology, 2023. 41(7): p. 907-922.
[5] Lutz, M.B., et al., Guidelines for mouse and human DC generation. European Journal of Immunology, 2022. 53(11).
[6] Ulrich, H., et al., A protocol for rapid monocyte isolation and generation of singular human monocyte-derived dendritic cells. Plos One, 2020. 15(4).
[7] Sontag, S., et al., Differentiation of Human Induced Pluripotent Stem Cells (iPS Cells) and Embryonic Stem Cells (ES Cells) into Dendritic Cell (DC) Subsets. Bio-Protocol, 2017. 7(15).
[8] Macri, C., et al., Dendritic cell subsets. Seminars in Cell & Developmental Biology, 2018. 84: p. 11-21.
[9] Subtil, B., et al., The Therapeutic Potential of Tackling Tumor-Induced Dendritic Cell Dysfunction in Colorectal Cancer. Frontiers in Immunology, 2021. 12.
[10] Eich, C., et al., Isolation and high-dimensional flow cytometric analysis of tumor-infiltrating leukocytes in a mouse model of colorectal cancer. Frontiers in Immunology, 2024. 15.
That's all for today's explanation, if you have any cell culture questions, welcome to join the group to communicate!
Note: The source of the picture network, invaded and deleted!
This issue is recommended by Xiaoai
|
Catalog number |
name of article |
specification |
|
Fetal bovine serum (Premium) |
500mL |
|
|
abs9484 |
RPMI 1640 medium (with L-glutamine, without HEPES) |
10mg |
|
abs9506 |
α-MEM medium (with nucleosides, with deoxynucleosides, with L-glutamine, with sodium pyruvate, without HEPES) |
500mL |
|
Penicillin-streptomycin solution (100 ×, bispecific antibody) |
100mL |
|
|
abs47048003 |
Collagenase type IV |
50ug |
|
abs47048304 |
Trypsin-EDTA digest (0.05% with phenol red) |
100mL |
|
abs47048305 |
Trypsin-EDTA digestion solution (0.05%, phenol red free) |
5mg |




