Dual Staining for p16Ki-67: A Precise Approach for the Diagnosis of Cervical Precancerous Lesions
05 Aug 2025
by AntBio
p16 is a tumor suppressor gene associated with cell cycle regulation. It functions by inhibiting the activity of CDK4 and CDK6, thereby preventing cells from entering the S phase. It plays a crucial role in the regulation of the G1-S transition in the cell cycle.
In cervical intraepithelial neoplasia (CIN), the expression level of p16 is abnormally elevated, characterized by abnormal nuclear and cytoplasmic positivity of the p16 protein. It can be used to differentiate high-grade squamous intraepithelial lesions from metaplasia and atrophy.
Ki-67 is a cellular proliferation marker. Ki-67 is expressed during the G1, S, G2, and M phases of the cell cycle but is not expressed when cells are in the quiescent phase (G0) or undergoing apoptosis.
The detection of Ki-67 has applications in the diagnosis and grading of various cancers, including breast cancer, lung cancer, and colorectal cancer. The level of Ki-67 directly reflects the proliferative activity of tumor cells and is an important marker for assessing tumor malignancy and prognosis.
Cervical Cancer
Cervical cancer is one of the most common gynecological malignancies, ranking second in incidence among female malignancies in China.
Persistent infection with high-risk human papillomavirus (HPV) is the primary cause of cervical cancer and cervical intraepithelial neoplasia, especially certain high-risk types, such as HPV 16 and HPV 18. High-grade cervical intraepithelial neoplasia includes high-grade squamous intraepithelial lesions (HSIL) and adenocarcinoma in situ (AIS). HSIL encompasses cervical intraepithelial neoplasia (CIN) grade 2 and grade 3, which are mainly caused by HPV infection and characterized by significant cellular atypia and increased mitotic figures.
Cervical cancer progresses slowly, often taking several years to develop from precancerous lesions to invasive cancer. Precancerous cervical lesions and early-stage cervical cancer may not present any symptoms. Given the suboptimal treatment outcomes and prognosis for cervical cancer, regular cervical cancer screening is crucial for early detection and treatment.
Traditional cervical cancer screening methods, such as cytology/HPV testing, have limitations in terms of specificity and sensitivity. In 2023, the "Chinese Expert Consensus on Cervical Lesion Screening and Management of Abnormalities in Elderly Women" included p16/Ki-67 dual staining in the screening protocol.
p16/Ki-67 Dual Staining
Dual Staining for p16/Ki-67 is an immunocytochemical method for the diagnosis of cervical precancerous lesions, using cervical cytology samples. The principle is that normal cells do not simultaneously overexpress p16 and Ki-67. Dual staining positivity indicates persistent infection with high-risk HPV and is associated with high-grade squamous intraepithelial lesions (HSIL).
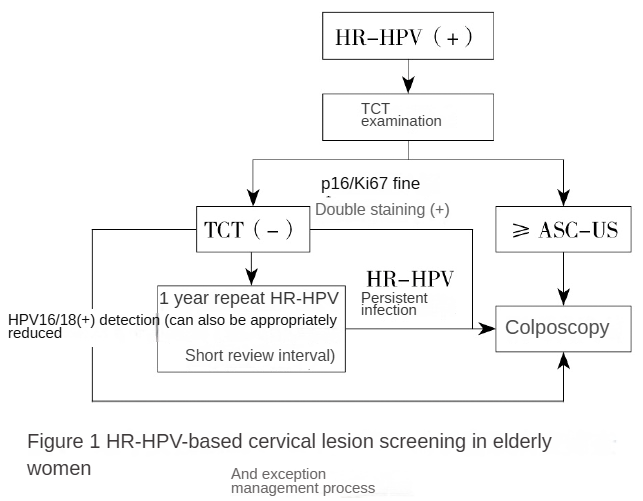
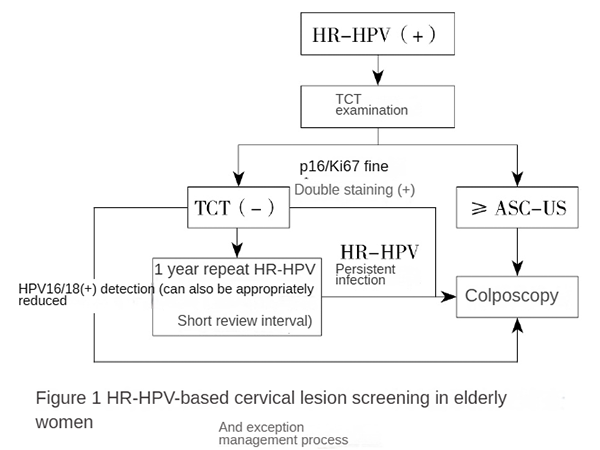
Figure 1: Screening and Management Flowchart for Cervical Lesions in Elderly Women Based on HR-HPV. HR-HPV: High-risk human papillomavirus; TCT: Thin-layer liquid-based cytology; ASC-US: Atypical squamous cells of undetermined significance.
Numerous studies have compared screening methods using clinical samples:
-
Mariam El-Zein Study: Collected 492 cervical samples for dual staining. Results showed that the positivity rate of dual staining cytology increased with the severity of histology, from 30.6% in normal tissue to 87.5% in cancer tissue. When used alone, dual staining cytology had lower sensitivity for CIN2+ and CIN3+ compared to HPV testing but increased specificity by 7.9% and 9.6%, respectively.
-
Chinese Clinical Sample Study: Selected cervical cytology samples from 537 women aged 20 to 79 years. The specificity of p16/Ki-67 in detecting CIN2+ and CIN3+ was higher than that of HR-HPV testing (85.96% vs. 67.54% for CIN2+, 79.84% vs. 62.90% for CIN3+). Dual staining can effectively triage women with ASC-US.
Diagnostic Advantages of p16/Ki-67 Dual Staining
In clinical practice, p16/Ki-67 dual staining has high sensitivity in cervical cancer screening, with both specificity and sensitivity superior to HPV and cytology testing. It helps increase the detection rate of cervical precancerous lesions and reduces unnecessary colposcopy.
p16/Ki-67 dual staining is not affected by different HPV subtypes and can independently assess the severity of lesions. The positivity rate of dual staining increases with the severity of cervical lesions, reducing the risk of delayed diagnosis.
Dual staining avoids localization differences caused by single staining. By locating positive cells in the squamous epithelium, combined with TCT and HPV testing, it can monitor the recurrence of HSIL (CIN II/III) and aid in the pathological diagnosis, grading, and stratified management of cervical CIN.
Dual staining is not influenced by cytological morphology. The cytoplasmic staining is specific brown (p16), and the nuclear staining is specific red (Ki-67). Diagnosis can be made based on color changes alone, providing a more objective and convenient method for the auxiliary diagnosis of cervical precancerous lesions, significantly improving both operational simplicity and accuracy.
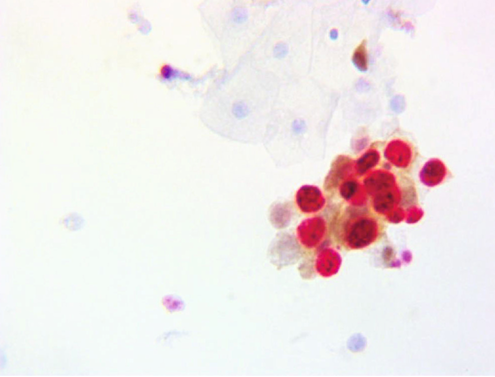
Figure 2: Positive Dual Staining of Cervical Epithelial Cells for p16/Ki-67
With the aging population and uneven socio-economic development in China, cervical lesion screening rates are low in some areas, and the incidence of cervical cancer is gradually increasing. Increasing screening coverage and optimizing screening methods are urgent needs. p16/Ki-67 dual staining technology offers a simpler, safer, and more efficient screening method for the early diagnosis of cervical cancer, providing strong support for early detection and treatment.
S-RMab® Monoclonal Antibodies
Starter's p16 & Ki-67 monoclonal antibodies are recombinant rabbit monoclonals with higher specificity, sensitivity, and stability. We provide accurate cellular localization and offer more accurate diagnostic results for clinical use.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B0001E | S-RMab® Ki67 Recombinant Rabbit mAb (SDT-10002) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B2259 | Ki67 Recombinant Mouse mAb (SDT-606-7) | Host : Mouse | Inquiry |
| Conjugation : Unconjugated |




