Unlocking the "Key" of Acetylation: Understanding Cellular Regulation and Cutting-edge Detection Strategies
16 Jun 2025
by AntBio
I. Acetylation: The Critical "Control Knob" of Biological Processes
Acetylation is a chemical modification where, under the catalysis of specific enzymes, an acetyl group (CH₃CO-) is linked to proteins, histones, non-histone proteins, or other molecules. This seemingly minor modification can profoundly alter the functional properties of molecules, much like a magical "wand." It can affect their activity levels, intracellular localization, structural stability, and interaction patterns with other molecules.
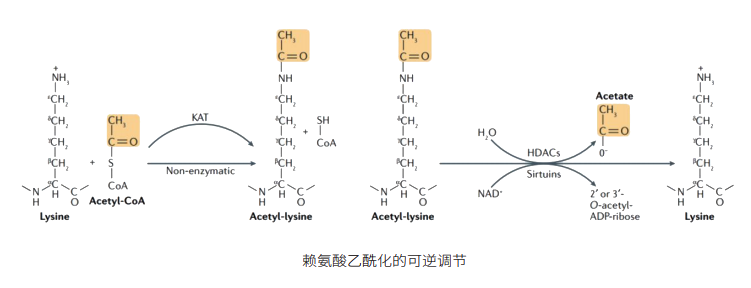
On the "stage" of proteins, acetylation acts as an excellent "regulator." It can flexibly modulate protein activity and subtly alter the binding affinity of proteins with other molecules, thereby reshaping the complex signaling pathways within cells. Taking histones as an example, the acetylation state of histones is closely linked to chromatin structure and gene expression. When histones are acetylated, chromatin structure becomes relaxed, opening the "gates" for gene expression and allowing transcription factors to access DNA freely, thereby promoting transcription. Conversely, when histones are deacetylated, chromatin structure tightens, closing the "valves" of gene expression and suppressing transcriptional activity.
The "influence" of acetylation extends far beyond this. It is also deeply involved in various critical biological processes, such as cellular metabolism, cell cycle regulation, and DNA damage repair. In the realm of cellular metabolism, acetylation acts like a precise "fine-tuning knob," finely regulating the activity of metabolic enzymes and maintaining the dynamic balance of cellular energy metabolism.
II. Acetylation Detection Strategies: Decoding Cellular "Passwords" with Cutting-edge Approaches
Given the central role of acetylation in biological activities, accurately measuring acetylation levels and modification sites has become a key component of life sciences research. Below is a detailed introduction to a widely used and effective acetylation detection strategy:
-
Sample Preparation
Depending on the research objective, appropriate biological samples (e.g., cells, tissues, or body fluids) are carefully collected. For cell samples, cell lysis techniques are used to release intracellular proteins, while tissue samples require homogenization to disperse the tissue into a cell suspension, followed by protein extraction. To preserve the native structure and biological activity of proteins, protease inhibitors are added during extraction, acting as a "protective armor" for proteins. -
Protein Separation
SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) is employed to separate the extracted proteins. This technique acts like a precise "molecular sieve," separating proteins into distinct bands based on their molecular weight, providing a clear "molecular map" for subsequent analysis. -
Western Blot Analysis
The separated proteins are transferred to a PVDF (polyvinylidene fluoride) or NC (nitrocellulose) membrane. Specific acetylation antibodies are then used to incubate with the membrane, forming antigen-antibody complexes. After washing to remove unbound antibodies, a secondary antibody labeled with fluorophore or enzyme is added. Finally, chemiluminescent or fluorescent detection techniques are used to sensitively detect acetylated proteins and analyze their relative expression levels. -
Mass Spectrometry for Precise Localization
To identify specific acetylation sites, mass spectrometry is the method of choice. Proteins are enzymatically digested into peptides, which are then separated and analyzed by mass spectrometry. By interpreting the mass spectra, researchers can precisely determine the presence and exact location of acetylation modifications, akin to pinpointing molecular "coordinates" in the microscopic world.
III. Acetylation Detection: Staart Acetylation Pan-Modification Antibody as the Optimal Choice
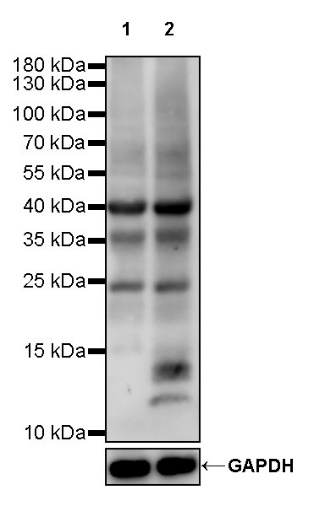
In the field of acetylation detection, Staart's Acetylation Pan-Modification Antibody (Acetyllysine Rabbit polyclonal antibody, Catalog No. S0B0655) stands out for its high specificity and sensitivity. Experimental results (WB result of Acetyllysine Rabbit mAb) show that when used at a 1:500 dilution as the primary antibody and a Goat Anti-rabbit IgG, (H+L), HRP conjugated secondary antibody at a 1:10,000 dilution, the antibody clearly detects acetylated proteins. Lane 1 contains untreated HeLa whole cell lysate (20 µg), and Lane 2 contains HeLa whole cell lysate (20 µg) treated with 500 ng/ml TSA for 4 hours. The antibody predicts multiple molecular weights, and multiple bands are observed, providing reliable data support for researchers.
Conclusion
As an indispensable chemical modification in biological systems, the development of acetylation detection technologies is invaluable for exploring life's mysteries, uncovering disease mechanisms, and developing innovative therapeutic strategies. With cutting-edge detection methods and high-quality tools, researchers can efficiently unlock the "acetylation key" within cells, driving the advancement of life sciences and aiding humanity in its quest to explore life's complexities.