TDP-43 and ALS: Guardian or Betrayer?

TDP-43 is a protein composed of 414 amino acids and belongs to the heterogeneous nuclear ribonucleoprotein (hnRNP) family. It contains two RNA recognition motifs (RRMs), which form a conserved three-dimensional structure through the folding of two 60-residue amino acid segments, enabling single-stranded and sequence-specific binding to RNA. This binding capability allows TDP-43 to participate in various intracellular RNA metabolic processes, including transcription, translation, alternative splicing, and mRNA stability regulation. The protein was first discovered by Virginia Lee in 2006, who revealed its critical role in neurodegenerative diseases.
Structure and Function
In normal cells, TDP-43 is primarily localized in the nucleus, where it regulates gene expression. However, under cellular stress conditions, TDP-43 can translocate to the cytoplasm and interact with other proteins to form stress granules, which help cells adapt to environmental changes. These stress granules are membraneless structures formed through reversible liquid-liquid phase separation, aiding in maintaining cellular homeostasis under stress.
In disease states, TDP-43 forms abnormal protein aggregates in the cytoplasm, a hallmark pathological feature of amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease) and frontotemporal lobar degeneration (FTLD). These aggregates are closely associated with the development and progression of ALS.
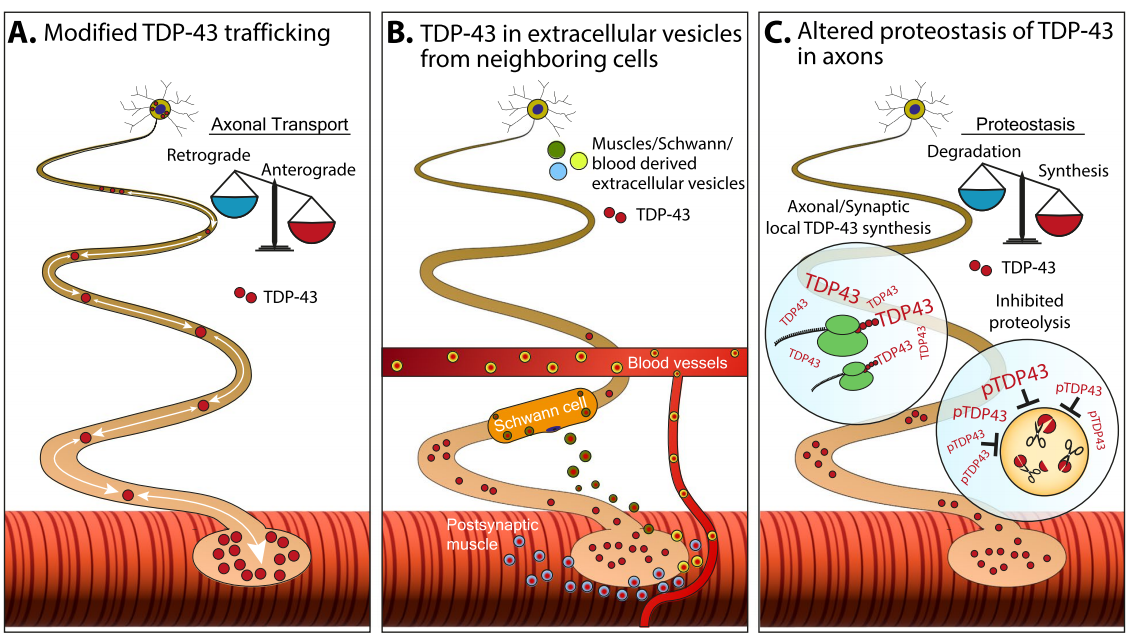
Figure 1. Mechanisms of TDP-43 Accumulation in Axons.
Schematic representation of potential mechanisms underlying the formation and accumulation of pathological TDP-43 in axons.
A. Altered axonal transport may shift TDP-43 toward increased anterograde or reduced retrograde transport, altering its local concentration in axons and synapses.
B. TDP-43 spreads via extracellular vesicles. One mechanism for TDP-43 clearance is through extracellular vesicles. These TDP-43-containing vesicles can be released from Schwann cells or skeletal muscle and taken up by neighboring axons, which may lack the capacity to handle protein overload and proper mechanisms to manage protein accumulation.
C. Dysregulation of protein homeostasis driven by unregulated TDP-43 synthesis in axons or impaired TDP-43 proteolysis and clearance due to its phosphorylation state.
TDP-43 and ALS
In ALS and FTLD patients, TDP-43 protein aggregates are abundantly detected in the spinal cord and affected brain regions. These aggregates primarily exist in an amyloid-like form and are closely associated with pathological features such as amyloid plaques, neurofibrillary tangles, and hippocampal sclerosis.
Research indicates that TDP-43 plays a critical role in ALS. In ALS patients, pathological abnormalities of TDP-43 are nearly universal, regardless of whether mutations in the TARDBP gene are present. These abnormalities include the translocation of TDP-43 from the nucleus to the cytoplasm and the formation of inclusion bodies, which disrupt TDP-43's normal functions, exert toxicity, and interfere with cellular processes, ultimately leading to cell death.
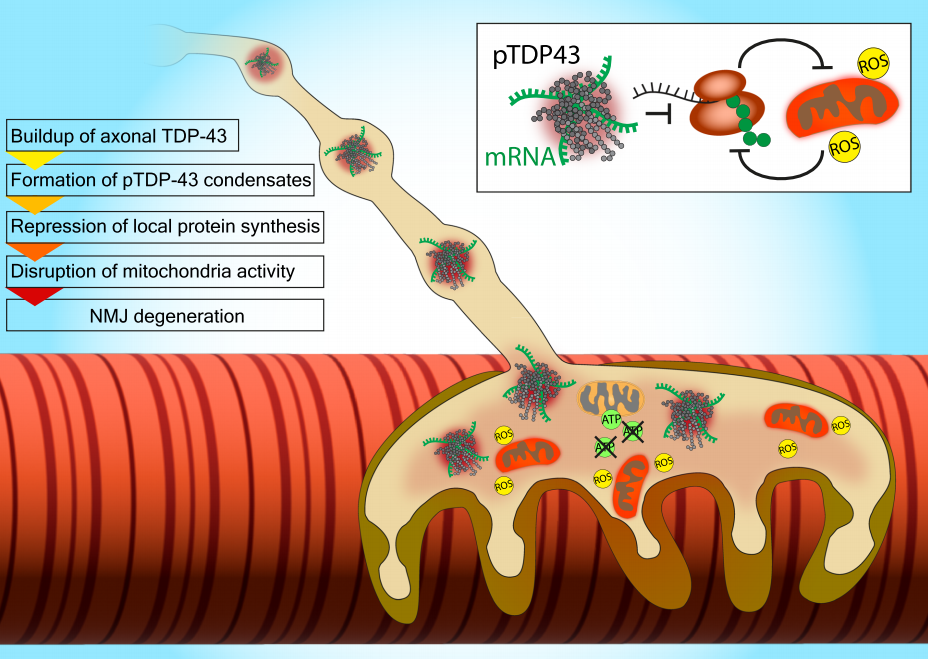
Figure 2. Proposed Pathological Mechanisms of TDP-43 in Axons/NMJ.
Emerging evidence on the biological and pathological roles of axonal TDP-43 suggests that TDP-43 accumulates in the distal axons and presynaptic compartments of motor neurons, forming pTDP-43 condensates. These structures sequester mRNAs critical for the local synthesis of mitochondrial and ribosomal proteins. Disruption of local protein synthesis triggers a vicious cycle of mitochondrial dysfunction, reactive oxygen species (ROS) production, and further impairment of local protein synthesis. This particularly affects the neuromuscular junction (NMJ), which heavily relies on these processes, thereby promoting NMJ and axonal degeneration.
Case Study
Researchers extracted aggregated TDP-43 from the frontal and motor cortices of two patients with a clinical history of ALS combined with FTLD. Both patients exhibited round neuronal cytoplasmic inclusions (NCIs) of TDP-43 in the motor cortex and spinal cord, along with characteristic features of sporadic ALS (Figure 3a, Extended Data Figure 3a). NCIs and oligodendroglial cytoplasmic inclusions were also observed across all cortical layers of the frontal, temporal, and parietal cortices, with few dystrophic neurites (Figure 3a, b, Extended Data Figure 3b). These findings align with stage 4 ALS TDP-43 pathology and type b cortical TDP-43 pathology, both commonly seen in ALS-FTLD patients. Immunoblotting confirmed that the extracted aggregates consisted of full-length TDP-43 and C-terminal fragments (CTFs), phosphorylated at S409 and S410 (pS409/S410) (Figure 3b, Extended Data Figure 3c), consistent with previous observations in ALS and FTLD. Immunogold-negative staining electron microscopy (immune-EM) revealed that TDP-43 aggregates formed filaments with helical projections 10-15 nm in width (Figure 3d, Extended Data Figure 3d), similar to TDP-43 aggregates extracted from FTLD patients and those imaged in situ in ALS and FTLD brain tissues.
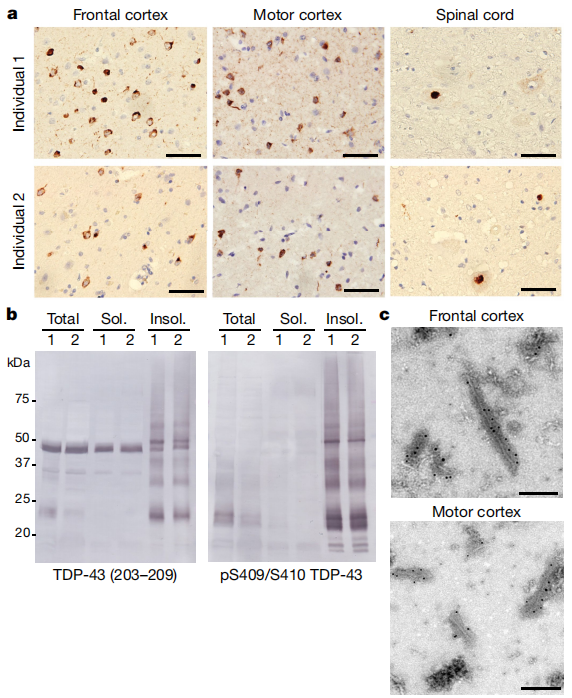
Figure 3 | TDP-43 Pathology of ALS with FTLD.
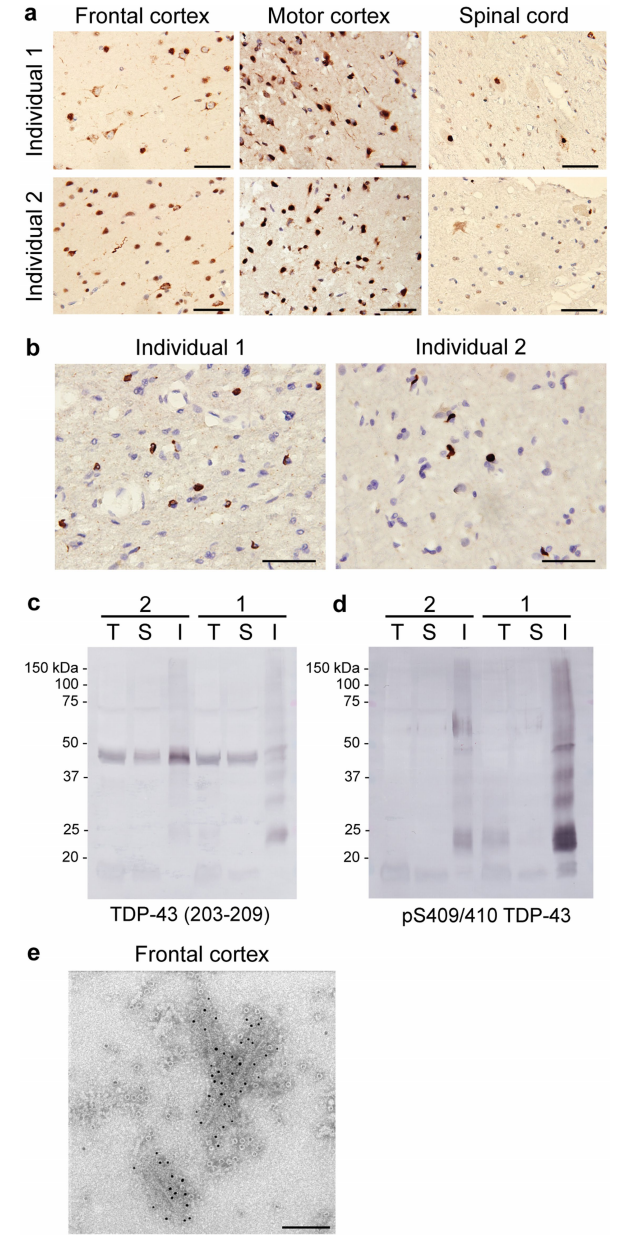
Extended Data Figure 3 | TDP-43 Pathology of ALS with FTLD Continued.
Recent studies have also found that abnormal expression or mutations in TDP-43 affect astrocyte function. Astrocytes, a critical cell type in the central nervous system, play roles in maintaining neuronal microenvironment stability, synaptic transmission, and neuroprotection. Research shows that abnormal TDP-43 expression in astrocytes promotes the expression of interferon-induced chemokine genes, leading to excessive chemokine production. This activates chemokine receptors in neurons, alters presynaptic function, and causes neuronal hyperexcitability, ultimately resulting in cognitive impairment.
TDP-43, as a multifunctional nucleic acid-binding protein, plays a significant role in neurodegenerative diseases. Its abnormal expression or mutations contribute to the development of various neurodegenerative disorders. Future research should further elucidate the specific mechanisms by which TDP-43 abnormalities lead to neurodegeneration and develop effective therapeutic agents targeting TDP-43. Additionally, early screening and diagnosis of neurodegenerative diseases should be strengthened to enable timely detection and treatment.
TDP-43 Recombinant Rabbit mAb
Data Sharing Of Catalog Number: S0B0731

ICC shows positive staining in HeLa cells. Anti-TDP-43 antibody was used at 1/500 dilution (Green) and incubated overnight at 4°C. Goat polyclonal Antibody to Rabbit IgG - H&L (Alexa Fluor® 488) was used as secondary antibody at 1/1000 dilution. The cells were fixed with 4% PFA and permeabilized with 0.1% PBS-Triton X-100. Nuclei were counterstained with DAPI (Blue). Counterstain with tubulin (Red).

IHC shows positive staining in paraffin-embedded human colon. Anti- TDP-43 antibody was used at 1/1000 dilution, followed by a HRP Polymer for Mouse & Rabbit IgG (ready to use). Counterstained with hematoxylin. Heat mediated antigen retrieval with Tris/EDTA buffer pH9.0 was performed before commencing with IHC staining protocol.

IHC shows positive staining in paraffin-embedded human testis. Anti- TDP-43 antibody was used at 1/1000 dilution, followed by a HRP Polymer for Mouse & Rabbit IgG (ready to use). Counterstained with hematoxylin. Heat mediated antigen retrieval with Tris/EDTA buffer pH9.0 was performed before commencing with IHC staining protocol.

WB result of TDP-43 Recombinant Rabbit mAb
Primary antibody: TDP-43 Recombinant Rabbit mAb at 1/1000 dilution
Lane 1: HeLa whole cell lysate 20 µg
Lane 2: SH-SY5Y whole cell lysate 20 µg
Secondary antibody: Goat Anti-Rabbit IgG, (H+L), HRP conjugated at 1/10000 dilution
Predicted MW: 45 kDa
Observed MW: 40 kDa

Flow cytometric analysis of 4% PFA fixed 90% methanol permeabilized HeLa (Human cervix adenocarcinoma epithelial cell) labelling TDP-43 antibody at 1/500 dilution (0.1 μg) / (Red) compared with a Rabbit monoclonal IgG (Black) isotype control and an unlabelled control (cells without incubation with primary antibody and secondary antibody) (Blue). Goat Anti - Rabbit IgG Alexa Fluor® 488 was used as the secondary antibody.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B0731 | TDP-43 Recombinant Rabbit mAb (S-989-41) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated |




