TAU biomarkers: precision diagnosis in Alzheimer's disease
Alzheimer's disease (AD) is a degenerative disease of the central nervous system characterized by progressive memory loss, cognitive decline, and behavioral and emotional disorders.
In 1907, Alois Alzheimer and Oskar Fischer observed plaques and tangles in the brain tissue of AD patients, thus opening the door to biomarkers of AD: plaques and tangles - the "amyloid cascade hypothesis" of AD pathogenesis.
The amyloid cascade hypothesis
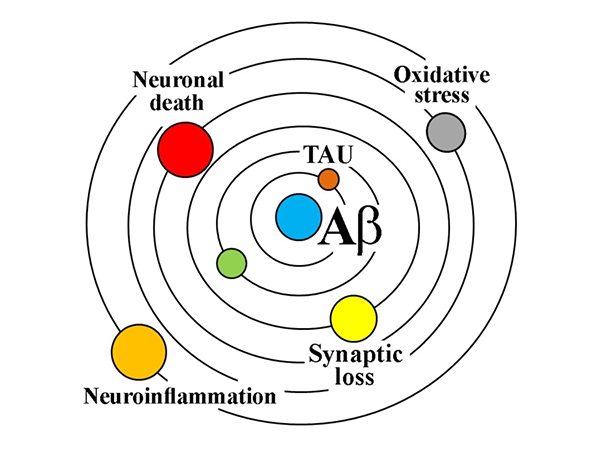
The cascade hypothesis suggests that as the main component of amyloid neuritis plaque, Aβ protein can be secreted and released into the intercellular fluid under the environment of enhanced neuronal activity, aggregated into oligomers and formed plaques. It also triggers hyperphosphorylation of Tau protein, leading to the formation of paired helical filaments and neurofibrillary tangles (NFTS), leading to a cascade of synaptic dysfunction, loss of glial cell activity, and neuronal death. Therefore, β-amyloid (Aβ) plaques and tau neurofibrillary tangles have become the two major pathological markers of AD, which are helpful for the accurate identification of AD in preclinical and even early stages.
Alzheimer's disease Diagnosis
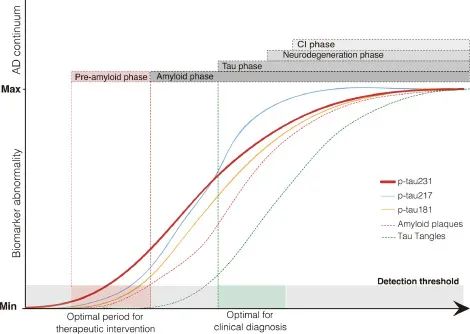
Aβ is thought to be the cause of AD, but its poor correlation with the later stages of AD requires more attention to downstream markers of neurodegenerative diseases, such as Tau. The diagnostic research framework for AD (ATN) proposed by the Alzheimer's Disease Association International Conference (AAIC) in 2018 points out that Aβ aggregation or related pathological state is indicated by A positive, which means that AD pathological changes occur. Tau aggregation or related pathological state is indicated by positive T, and the diagnosis of AD can only be made if both biomarkers A and T are positive.
At this time, the generally accepted and recognized detection methods are Cerebro-Spinal Fluid (CSF) examination and positron emission tomography (PET), but CSF examination is invasive, and PET scan is expensive and easy to accumulate radiation. Liquid biopsy, which is low-cost, simple to operate and easy to popularize, has gradually entered the field of vision.
In July 2023, the AAIC added blood biomarkers to the AD core markers. Plasma Tau can reflect the state of neurodegeneration and blood-brain barrier damage in the process of the disease, and it also has higher accuracy than Aβ in diagnosis. Meanwhile, tau from plasma and CSF has a structurally similar phase pattern, which also ensures that some p-tau in CSF is detected at the same level as p-tau in plasma. The Chinese expert consensus on simple screening for prodromo Alzheimer's disease (2023 version) also clearly states that screening blood p-tau in clinical diagnosis and treatment has a certain predictive value for the progression of early AD patients.
Tau
Tau protein is considered as a biomarker of axonal degeneration and neurofibrillary tangle formation in Alzheimer's disease, reflecting nonspecific changes in neuronal secretion of tau and cortical thickness, but is not a direct biomarker of neuronal loss [2]. In the adult brain, there are six isoforms of tau.
tau is susceptible to post-translational modification (PTM), including phosphorylation, acetylation, glycosylation, etc., which leads to the reduction of microtubule binding ability. Tau protein contains 79 potential serine and threonine phosphorylation sites and is associated with different diseases. In addition to modifications, tau concentrations in CSF of AD patients are as high as 900 ng/mL, much higher than 300 ng/mL of healthy people, and several studies have shown that t-tau sensitivity and specificity in AD patients are 80-90% [3]. Due to the complexity of the modification and the diversity of its isoforms, the analysis and detection of each molecule is still a great challenge. Plasma/CSF levels of total tau and phosphorylated tau have been detected using well-established methods. p-tau217, p-181, and p-231 have high specificity and translational value.
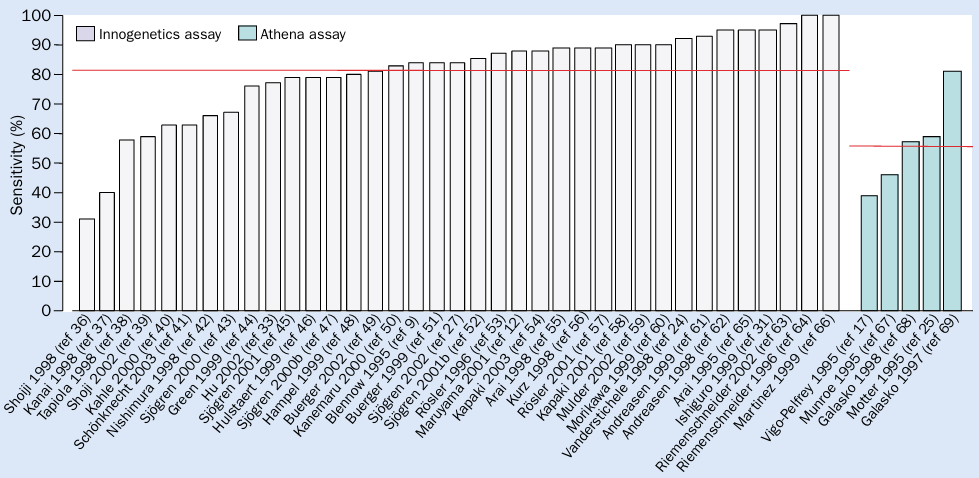
Figure 2 CSF t-tau as a diagnostic marker for AD. The red line indicates the mean sensitivity compared to the control group.
p-Tau 217
In July 2023, the AAIC first recommended plasma p-tau217 as a core marker for the diagnosis of AD. Plasma p-tau217 had excellent diagnostic accuracy for AD by either Aβ-PET or tau-PET (area under the curve, 0.87 or 0.93, P<0.001; There was no significant difference between plasma p-tau217 and CSF p-tau217 [4]. Different from the inability of tau-PET to detect insoluble tau aggregates in early clinical patients, p-tau217 can be detected in plasma [5]. Moreover, some studies have found that p-tau217 in plasma can also induce tau hyperphosphorylation at other sites, aggravating tau fibrosis and cognitive impairment [6].
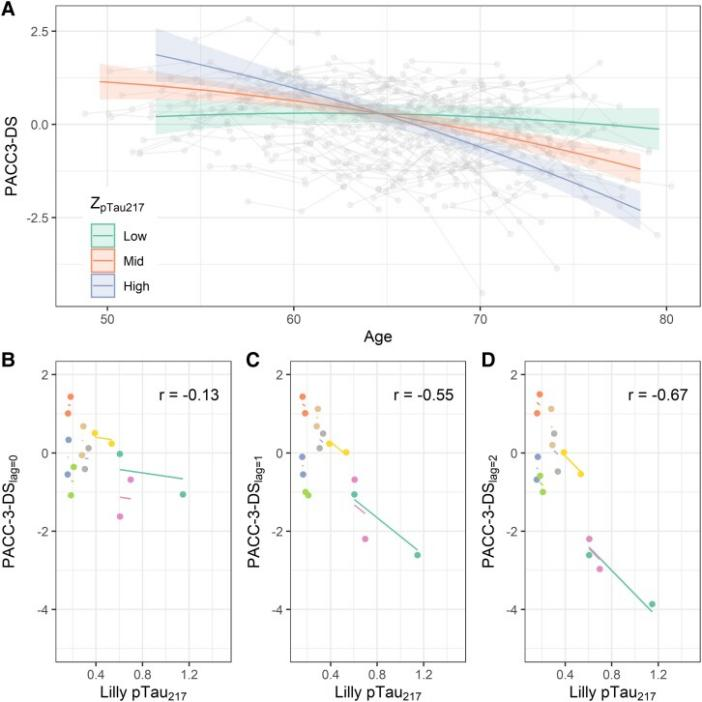
Figure 3 Association of P-tau217 with longitudinal cognition. The steepest slope of panel D indicates a stronger inverse association between early p-tau217 and preclinical AD composite cognition.
p-Tau 181
P-tau181 is also a highly specific pathological marker of AD, and the AUC value of plasma p-tau181 is also statistically significant, which can significantly distinguish AD from other neurodegenerative diseases. Many studies have shown that plasma p-tau181 concentration is positively correlated with the progression of AD, and plasma p-tau181 concentration is significantly higher in AD individuals than in individuals with mild cognitive impairment (MCI). Plasma p-tau181 can be used as a marker for the pathological progression of AD. p-tau181 has a good performance in the early identification, diagnosis and prognosis of AD [7], [8].
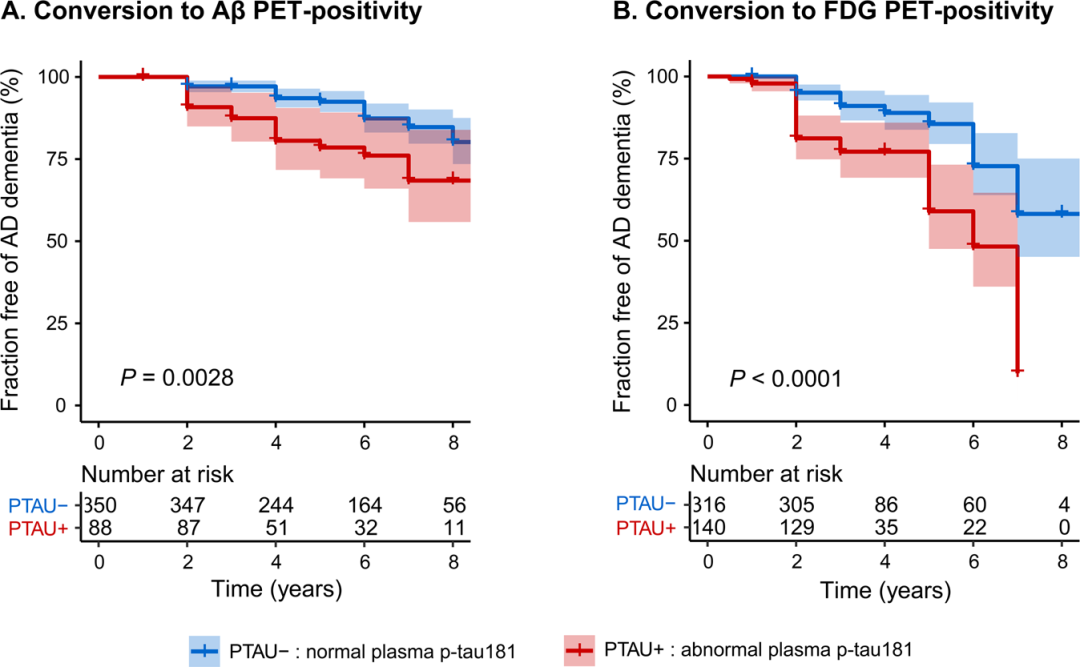
Figure 4 Relationship between baseline plasma p-tau181 levels and risk of pathological progression
p-Tau 231
Both CSF and plasma p-tau231 can be used as biomarkers for pathological and clinical diagnosis of AD.
CSF p-tau231 was the earliest clinically occurring biomarker to reach abnormal values. CSF p-tau231 has been shown to be the most relevant biomarker for uptake of the 18F-labeled Aβ-PET ligand ([18F] AZD4694) (TCU->3.19; PCU-<0.05), and the related regions were very concentrated, including the medial orbitofrontal cortex, precuneus cortex, and posterior cingulate cortex, which were better than P-181 and P-217. By analyzing the results of early Aβ metabolic abnormalities by polynomial regression, it was found that the abnormal inflection point of CSF p-tau231 was much earlier than that of p-tau181 and p-tau217, indicating that with the accumulation of Aβ, p-tau231 first showed abnormal levels [9].
Similarly, studies have tested the effect of plasma p-tau231 and [18F] AZD4694 intake, and the results were consistent with CSF p-tau231, and the inflection point of plasma p-tau231 was positively correlated with its intake and earlier than p-181 [10].
Figure 5 P-tau231 as a biomarker at the earliest stages of AD pathology
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B3157 | Tau (phospho T181) Mouse mAb (SDT-200-9) | Host : Mouse | $835 |
| Conjugation : Unconjugated | |||
| S0B3156 | Tau (phospho T181) Mouse mAb (SDT-200-5) | Host : Mouse | $835 |
| Conjugation : Unconjugated | |||
| S0B3099 | Tau (phospho T181) Recombinant Rabbit mAb (SDT-R045) | Host : Rabbit | $835 |
| S0B3173 | Tau (phospho T217) Recombinant Rabbit mAb (SDT-R205-TT217-4) | Host : Rabbit | $835 |
| Conjugation : Unconjugated | |||
| S0B3172 | Tau (phospho T217) Recombinant Rabbit mAb (SDT-176-13) | Host : Rabbit | $835 |
| Conjugation : Unconjugated | |||
| S0B3059 | Tau Recombinant Rabbit mAb (SDT-171-45) | Host : Rabbit | $835 |
| S0B3216 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-17) | Host : Rabbit | $835 |
| Conjugation : Unconjugated | |||
| S0B3217 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-1) | Host : Rabbit | $835 |
| Conjugation : Unconjugated | |||
| S0B3220 | Tau (phospho T231) Mouse mAb (SDT-202-2) | Host : Mouse | $835 |
| Conjugation : Unconjugated |