STARTER Pathological IHC Antibodies Facilitate Research on APOE+ Tumor-Associated Macrophages in the Hepatocellular Carcinoma Immune Microenvironment
The study titled APOE deficiency triggers anti-tumour activity of macrophages in liver cancer was published in the Nature sub-journal Cancer Gene Therapy. Led by Xintong Xia from Zhejiang Chinese Medical University, this research systematically investigates the function of APOE+ tumor-associated macrophages in the immune microenvironment of hepatocellular carcinoma and their potential as a new target for immunotherapy.

Experimental Background:
Hepatocellular carcinoma (HCC) is one of the malignant tumors with high incidence and mortality worldwide. However, immune checkpoint inhibitor (ICI) therapy shows limited efficacy in some patients. Tumor-associated macrophages (TAMs) in the tumor microenvironment play a crucial role in promoting tumor progression, angiogenesis, fibrosis, and immune suppression. TAMs exhibit high heterogeneity, with distinct functions among different subsets, and there is currently a lack of a unified and reproducible definition for these subsets. Apolipoprotein E (APOE) is abnormally expressed in various tumors and is closely associated with immune cell infiltration and cholesterol metabolism. Nevertheless, its specific role in TAMs of HCC remains unclear.
Research Approach:
(I) Research Objective: Identify key immunosuppressive macrophage subsets in HCC and their clinical significance
Experimental Methods:
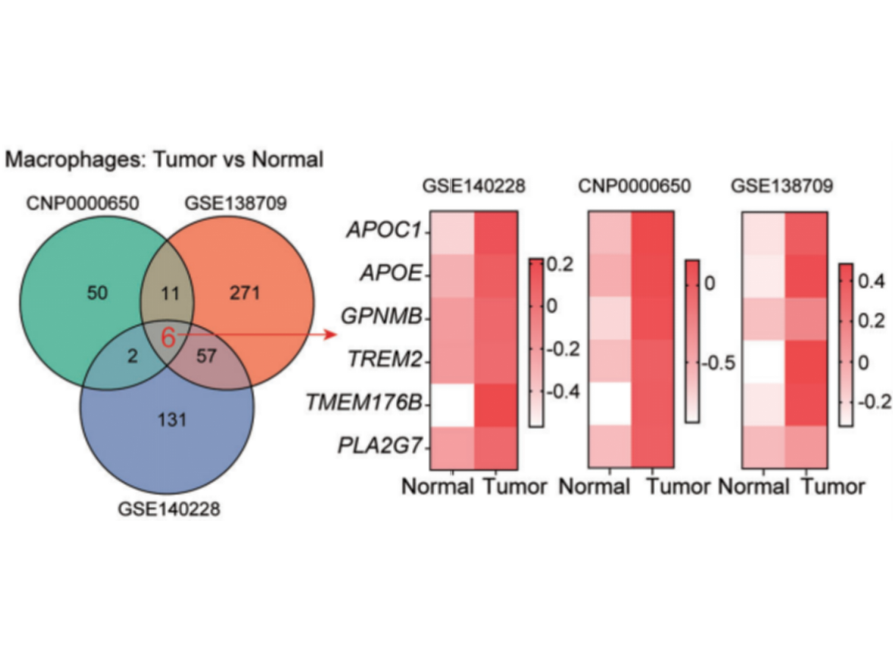
1. Integrate and re-analyze single-cell RNA sequencing (scRNA-seq) data of HCC from multiple public databases (GSE140228, CNP000050, GSE138709).
2. Use Seurat and Harmony software for cell clustering, identification of differentially expressed genes (DEGs), and batch effect correction.
3. Perform survival analysis using the TCGA-LIHC cohort.
4. Verify protein expression in human HCC tissue samples through immunofluorescence (IF) staining.
Key Findings:
- A macrophage subset characterized by high APOE expression (APOE+ TAMs) was found to be enriched in tumor tissues.
- The gene signature of APOE+ TAMs is significantly associated with poor overall survival in HCC patients.
- The abundance of APOE+ TAMs is higher in tumors of non-responders to immune checkpoint blockade (ICB) therapy.

Figure 1 APOE is upregulated in TAMs in liver cancer
(II) Research Objective: Elucidate the functional characteristics and potential mechanism of action of APOE+ TAM
Experimental Methods:
1. Employ Gene Set Enrichment Analysis (GSEA) and Gene Set Variation Analysis (GSVA) to explore the biological pathways of APOE+ TAMs.
2. Use SCENIC analysis to infer key transcription factors regulating APOE+ TAMs.
3. Induce bone marrow-derived macrophages (BMDMs) in vitro using conditioned medium (TCM) from the mouse HCC cell line Hepa 1-6 to simulate the TAM phenotype.
4. Evaluate the expression of APOE, cholesterol metabolism-related genes (Abca1, Abcg1), and cellular cholesterol content via qPCR, immunofluorescence, and cholesterol detection kits.
5. Knock down the Apoe gene in BMDMs using siRNA to verify its function.
Experimental Results:
- APOE+ TAMs are significantly enriched in lipid metabolism and cholesterol efflux pathways.
- NR1H3 (LXRα) was identified as an active key transcription factor in APOE+ TAMs.
- TCM successfully induces high Apoe expression in BMDMs and enhances their cholesterol efflux capacity (manifested by upregulated Abca1/Abcg1 and decreased total cholesterol content), and this effect is attenuated after Apoe knockdown.

(III) Research Objective: Verify the in vivo effect of APOE+ TAMs on tumor growth and CD8+ T cell function
Experimental Methods:
1. Establish a mouse subcutaneous tumor-bearing model: co-inject Hepa 1-6 cells with BMDMs transfected with control (scramble) siRNA or Apoe-knockdown (siApoe) siRNA.
2. Analyze the infiltration of immune cells in tumors using flow cytometry and immunofluorescence staining.
3. Evaluate tumor growth status by detecting Ki67 (a proliferation marker) via immunohistochemistry (IHC) (STARTER Cat. No.: S0B2332) and TUNEL staining (an apoptosis marker).
Experimental Results:
- Compared with the control group, tumor growth in mice injected with Apoe-knockdown BMDMs was significantly slowed down.
- The infiltration of CD8+ T cells in tumors of the Apoe-knockdown group was significantly increased, while there was no significant change in CD4+ T cells.
- In the Apoe-knockdown group, tumor cell proliferation (Ki67+) was reduced, and apoptosis (TUNEL+) was increased.
 Figure 2 APOE+ TAMs inhibit HCC tumour growth through the immunomodulation of CD8+ T cells
Figure 2 APOE+ TAMs inhibit HCC tumour growth through the immunomodulation of CD8+ T cells
(IV) Research Objective: Explore the intercellular communication mechanism underlying ICB resistance induced by APOE+ TAMs
Experimental Methods:
1. Perform cell interaction analysis (CellChat) on scRNA-seq data (GSE206325) from ICB therapy responders and non-responders.
2. Co-culture T cells with control or Apoe-knockdown BMDMs in vitro, and detect the expression of T cell exhaustion markers (Pdcd1, Lag3, Tim3) via qPCR.
3. Treat T cells directly with cholesterol to observe its effect on the expression of exhaustion markers.
Experimental Results:
- In non-responders, the communication between APOE+ TAMs and CD8+ T cells (especially the exhausted subsets) is enhanced, involving signaling pathways such as MIF, GALECTIN-9, and CXCL12.
- After co-culture with Apoe-knockdown BMDMs, the expression of exhaustion markers in T cells decreased.
- Exogenous cholesterol treatment can induce T cells to express exhaustion markers.
 Figure 3 APOE+ TAMs are enriched in ICB non-responder tumours
Figure 3 APOE+ TAMs are enriched in ICB non-responder tumours
(V) Research Objective: Evaluate the potential of combined therapy targeting APOE and anti-PD-1
Experimental Methods:
1. Establish an immune-tolerant mouse model of spontaneous HCC induced by c-Myc/NRASV12.
2. Divide the mice into groups and administer the following treatments respectively: control agent, anti-PD-1 antibody, APOE inhibitor (COG133TFA), and combination of anti-PD-1 antibody and APOE inhibitor.
3. Count the number and size of liver tumor nodules via H&E staining, and analyze CD8+ T cell infiltration, cell apoptosis, and proliferation through IF and IHC.
Experimental Results:
- Anti-PD-1 monotherapy showed limited efficacy, while APOE inhibitor monotherapy had a slight effect.
-The combined therapy of anti-PD-1 antibody and APOE inhibitor exhibited a significant synergistic effect, which most effectively inhibited tumor growth, reduced tumor nodules, promoted CD8+ T cell infiltration, increased tumor cell apoptosis, and suppressed proliferation.

Figure 4 APOE inhibitor enhances the ICB efficacy of HCC
In this study, STARTER IHC antibody—Ki67 recombinant rabbit monoclonal antibody (Cat. No.: S0B2332)—was used for immunohistochemical experiments.
STARTER Popular Antibodies for Macrophage Research
|
Catalog No. |
Product Name |
Host Species |
Reactivity |
Application |
|
CD163 Recombinant Rabbit mAb (SDT-222-171) |
Rabbit |
Hu |
IHC-P, FCM, IF |
|
|
CD163 Recombinant Rabbit mAb (SDT-327-58) |
Rabbit |
Hu, Ms, Rt |
IHC-P, WB |
|
|
S-RMab® CD163 Recombinant Rabbit mAb (SDT-R164) |
Rabbit |
Hu, Ms, Rt |
IHC-P, WB, IP, IF |
|
|
CD11b Recombinant Rabbit mAb (SDT-058-44) |
Rabbit |
Hu |
IHC-P, ICC, WB, IP, ICFCM |
|
|
CD14 Recombinant Rabbit mAb (S-395-8) |
Rabbit |
Hu |
FCM |
|
|
ICAM-2 Recombinant Rabbit mAb (S-R359) |
Rabbit |
Hu |
IHC-P, ICC, WB, ICFCM |
|
|
ICAM-1/CD54 Recombinant Rabbit mAb (S-906-48) |
Rabbit |
Hu |
IHC-P, WB, FCM |
|
|
CD68 Recombinant Rabbit mAb (S-3394) |
Rabbit |
Ms, Rt |
IHC-P, ICC, WB |
STARTER Popular Antibodies for Hepatocellular Carcinoma Research
|
Catalog No. |
Product Name |
Host Species |
Reactivity |
Application |
|
S-RMab® CK-Pan Recombinant Rabbit mAb (SDT-P001) |
Rabbit |
Hu |
IHC-P, WB, IF |
|
|
CK7/8 Recombinant Rabbit mAb (SDT-P006) |
Rabbit |
Hu |
IHC-P |
|
|
CD10 Mouse mAb (S-713-5) |
Mouse |
Hu |
ICC, FCM |
|
|
CD10 Recombinant Rabbit mAb (SDT-006-77) |
Rabbit |
Hu, Ms, Rt |
IHC-P, WB, IP, IF |
|
|
AFP Recombinant Rabbit mAb (SDT-R031) |
Rabbit |
Hu, Ms |
IHC-P, ICC, WB |




