(pan)-Specific Antibodies and Custom Services: Facilitating Research on Post-Translational Modifications (PTMs)

Recent Advances
Protein post-translational modifications (PTMs) refer to the enzymatic addition or removal of chemical groups on amino acid residues after protein translation, mediated by various enzymes such as kinases and phosphatases. These modifications alter protein structure, function, stability, and interactions. To date, over 400 types of PTMs have been identified, with common examples including phosphorylation, glycosylation, methylation, ubiquitination, and acetylation. These modifications play critical roles in cellular signal transduction, regulation of gene expression, protein stability, and processes such as cell cycle progression and differentiation.
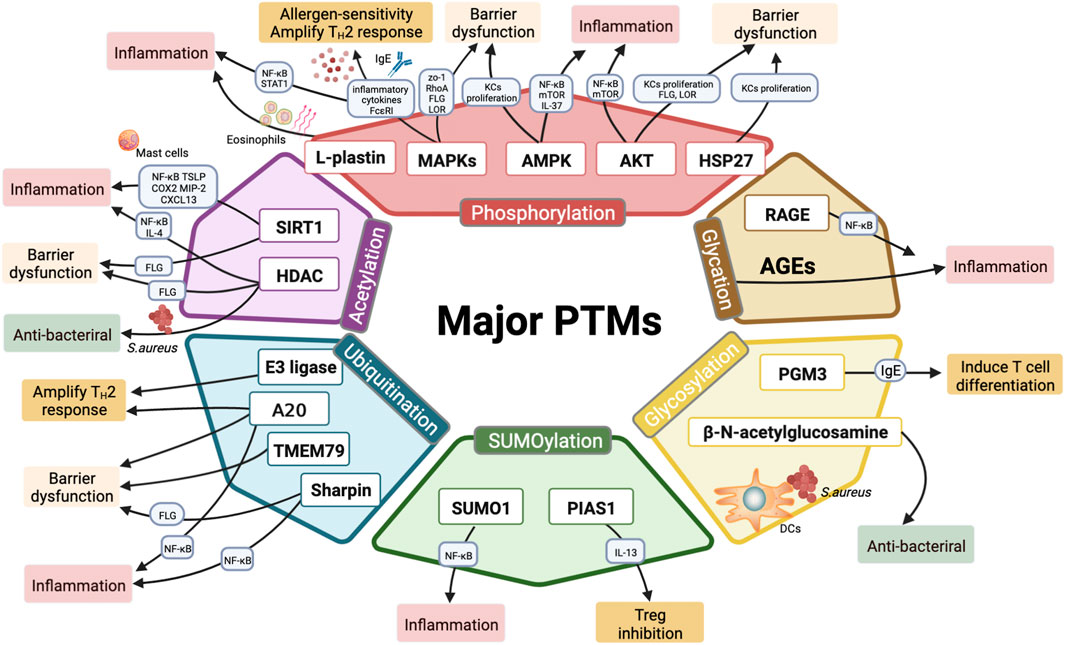
https://doi.org/10.3389/fcell.2022.942838
Common PTMs
Ubiquitination
Ubiquitination involves the covalent attachment of the small protein ubiquitin (Ubiquitin) to target proteins through a series of enzymatic reactions, including ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2, and ubiquitin ligase E3, forming ubiquitin chains. Ubiquitin, with a molecular weight of approximately 8.5 kDa, is ubiquitously present in all eukaryotic cells. This process can lead to protein degradation, signal transduction, cell cycle regulation, autophagy, endocytosis, DNA repair, and other biological effects.
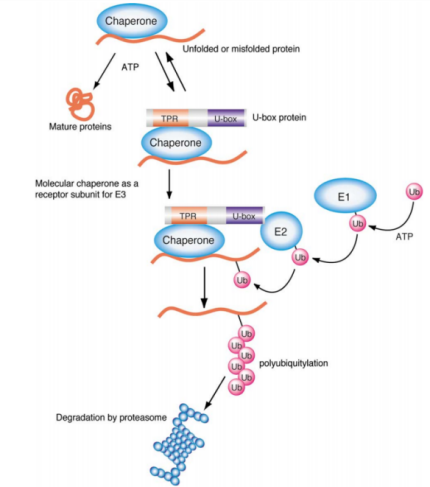
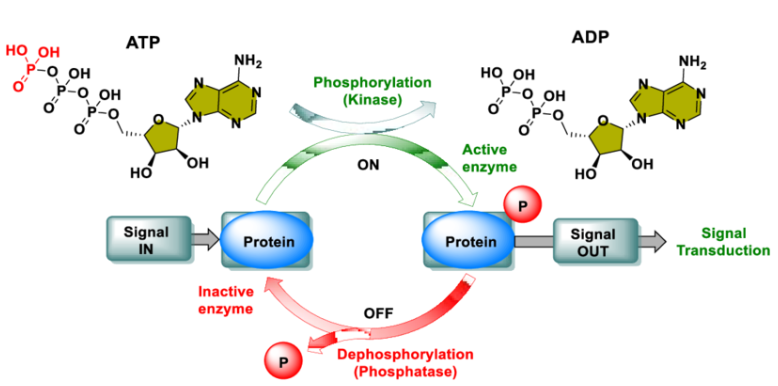
Phosphorylation
Phosphorylation involves the addition of phosphate groups to specific amino acid residues (serine, threonine, or tyrosine) through the catalytic action of kinases, thereby regulating protein activity, stability, interactions, and subcellular localization. It plays a crucial role in cellular signal transduction, cell cycle regulation, energy metabolism, and other biological processes. Dysregulation of phosphorylation is closely associated with the development of various diseases, including cancer and neurodegenerative disorders.
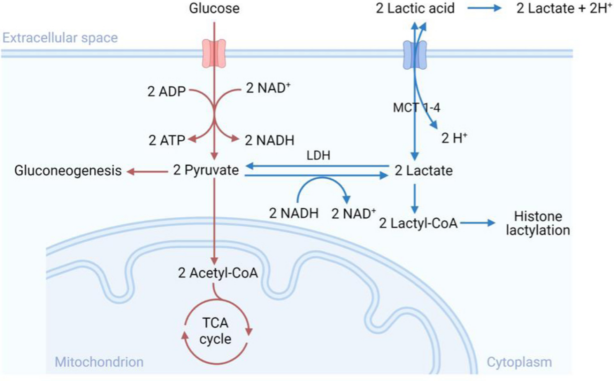
Lactylation
Lactylation refers to the formation of an amide bond between the carboxyl group of lactate and the amino acid residues of proteins (typically the ε-amino group of lysine). This process can alter the charge state and hydrophilicity of proteins, affecting their stability, conformation, activity, and intracellular localization. Lactylation may play a significant regulatory role in cellular metabolism and disease development, particularly under hypoxic conditions.
4-HNE Modification
4-Hydroxynonenal (4-HNE) modification is an important PTM that typically occurs on the side chains of cysteine, histidine, lysine, and some arginine residues in proteins. 4-HNE is a major product of lipid peroxidation and is highly toxic. It can react with proteins to form adducts, thereby affecting protein function. 4-HNE is a significant biomarker of lipid peroxidation and accumulates in large quantities during ferroptosis.
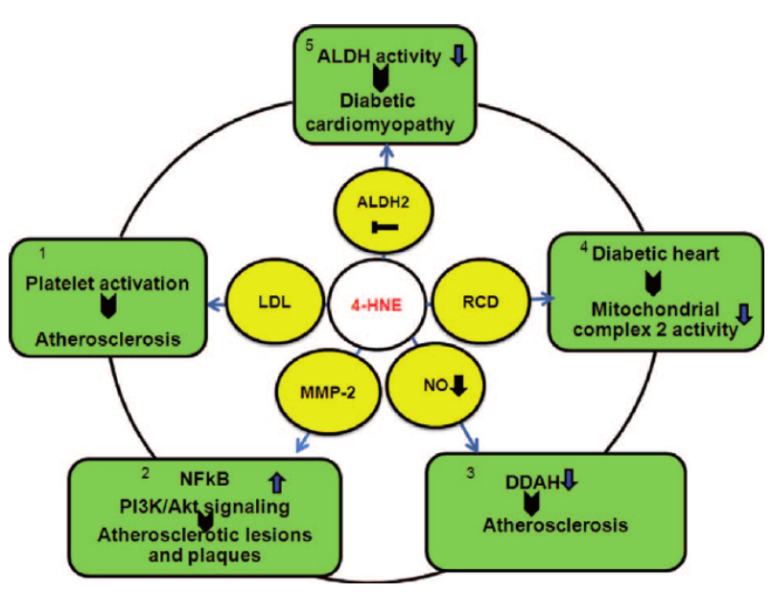
PTMs and Diseases
PTMs are associated with a variety of diseases, including metabolic disorders, cardiovascular diseases, neurodegenerative diseases, and inflammatory diseases.
-
Diabetes: PTMs influence insulin secretion in pancreatic β-cells by regulating signaling cascades, thereby contributing to the development of diabetes.
-
Non-Alcoholic Fatty Liver Disease (NAFLD): PTMs participate in the pathogenesis of NAFLD by modulating lipid synthesis, lipolysis, and fatty acid β-oxidation. The E3 ubiquitin ligase TRIM56 delays NAFLD progression by ubiquitinating the key fatty acid synthesis protein FASN.
-
Atherosclerosis: PTMs such as phosphorylation and acetylation affect the activity of key proteins in lipid metabolism pathways, potentially leading to dyslipidemia and promoting atherosclerosis. Acetylation regulates the process of atherosclerosis by modulating the acetylation and deacetylation of histones and nuclear proteins.
-
Neurodegenerative Diseases: Aberrant PTMs may lead to protein misfolding and aggregation. In Alzheimer's disease, abnormal phosphorylation of tau protein is associated with the formation of neurofibrillary tangles, a hallmark of the disease.
-
Inflammatory Responses: Phosphorylation can activate or inhibit key transcription factors in inflammatory signaling pathways, while acetylation may regulate the activity of histones and non-histone proteins, thereby influencing the transcription of inflammation-related genes. Dysregulated PTMs can lead to an imbalance in inflammatory processes.
S-RMab® PTMs Antibodies
Starter provides a variety of pan-specific antibodies, protein modification antibodies, and custom antibody services!
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B0332 | Propionyl-Histone H4 (Lys5) Recombinant Rabbit mAb (S-R093) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0054 | Tau (phospho T231) Recombinant Rabbit mAb (SDT-177-1) | Host : Rabbit | $350 |
| S0B0029 | Tau (phospho T181) Recombinant Rabbit mAb (SDT-R045) | Host : Rabbit | $100 |
| S0B0462 | Tau (phospho S396) Recombinant Rabbit mAb (S-R276) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0285 | RNA polymerase II CTD repeat YSPTSPS (phospho S7) Recombinant Rat mAb (S-R198) | Host : Rat | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0284 | RNA polymerase II CTD repeat YSPTSPS (phospho S2) Recombinant Rat mAb (S-R199) | Host : Rat | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0748 | Phospho-Stat1 (Tyr701) Recombinant Rabbit mAb (S-601-78) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0282 | Phospho-S6 Ribosomal Protein (Ser235/236) Recombinant Rabbit mAb (S-R203) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0784 | Phospho-p70 S6 Kinase (Ser371) Recombinant Rabbit mAb (S-1239-6) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0597 | Phospho-mTOR (Ser2448) Recombinant Rabbit mAb (S-705-7) | Host : Rabbit | $100 |
| Conjugation : Unconjugated | |||
| S0B0257 | Phospho-IκBα (Ser36) Recombinant Rabbit mAb (S-R102) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0631 | Phospho-IκBα (Ser32/36) Recombinant Rabbit mAb (S-751-40) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0518 | Phospho-GSK-3β (Ser9) Recombinant Rabbit mAb (S-748-20) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0596 | Phospho-Akt (Ser473) Recombinant Rabbit mAb (S-763-7) | Host : Rabbit | $100 |
| Conjugation : Unconjugated | |||
| S0B0611 | Phospho-Akt (Ser473) Recombinant Rabbit mAb (S-622-64) | Host : Rabbit | $100 |
| Conjugation : Unconjugated | |||
| S0B0363 | Phospho-Akt (Ser473) Recombinant Rabbit mAb (S-510-64) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0789 | Phospho-Histone H3 (Ser28) Recombinant Rabbit mAb (S-1025-45) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0751 | Phospho-Histone H3 (Ser10) Rabbit Polyclonal Antibody | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0296 | Histone H3 (mono methyl K36) Recombinant Rabbit mAb (S-R211) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0087 | Ubiquitin Recombinant Rabbit mAb (SDT-R095) | Host : Rabbit | $100 |
| S0B0737 | 4-Hydroxynonenal Recombinant Mouse mAb (S-R405) | Host : Mouse | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0373 | O-Linked N-Acetylglucosamine Recombinant Rabbit mAb (S-R256) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0735 | Phosphotyrosine Recombinant Mouse mAb (S-R433-2) | Host : Mouse | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0749 | Phosphotyrosine Recombinant Mouse mAb (S-R433-1) | Host : Mouse | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0319 | Phosphotyrosine Recombinant Rabbit mAb (S-R207) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0719 | L-Lactyl Lysine Rabbit Polyclonal Antibody | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0740 | Butyryllysine Recombinant Rabbit mAb (S-R399) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated | |||
| S0B0655 | Acetyllysine Rabbit polyclonal antibody | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated |




