Indispensable AAV Antibodies in Gene Therapy

Adeno-associated virus (AAV) is characterized by its low pathogenicity, low immunogenicity, and high gene delivery efficiency, making it a widely utilized viral vector in the field of gene therapy. AAV-based gene therapy is currently the only in vivo gene therapy approved in the United States and Europe.
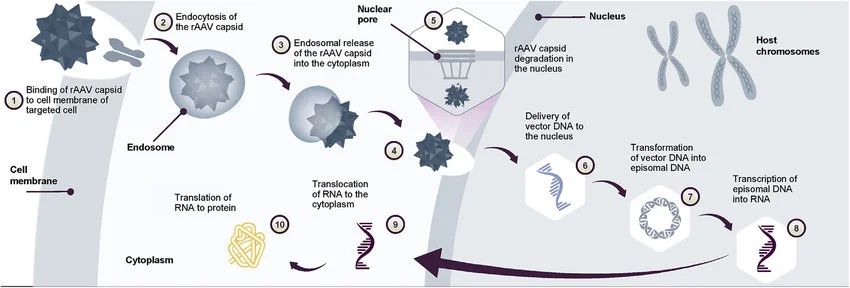
Figure 1: AAV Therapy
AAV vectors offer several advantages, including the fact that wild-type AAV has not been associated with any disease, the ability of AAV to achieve long-term expression of payloads in various cell types, and its lack of strong immunogenicity. To date, over 70% of gene delivery therapeutics employ AAV vectors, which are particularly well-suited for targeting tissues such as the liver, retina, and central nervous system.
AAV2
AAV serotypes are classified based on differences in the surface antigens of their capsid proteins. Dozens of AAV serotypes with distinct capsid proteins have been identified, each exhibiting unique spatial structures, sequences, and tissue specificities. These serotypes elicit different antisera responses in vivo.

Figure 2: Three-Dimensional Structure of AAV (BCRF PDB ID: 1LP3)
Among these, AAV2 is the most common serotype, sharing high amino acid sequence similarity with other serotypes. Nearly all recombinant AAV viral vectors are derived from the AAV2 subtype, making AAV2 the most extensively studied serotype to date.
AAV2 is a small, non-enveloped virus with a diameter of approximately 20-25 nm, classified as a type I pneumotropic virus. Its capsid is composed of 60 subunits, primarily consisting of three polypeptides—VP1, VP2, and VP3—which together form a protective shell for its genetic material.
AAV2 can efficiently transduce various cell types. Its low immunogenicity allows for prolonged in vivo expression, while its high transduction efficiency and broad tissue specificity make it an ideal vector for delivering therapeutic genes to specific cells. Over 200 AAV-related clinical trials are currently underway or nearing completion. For example, recombinant human adenovirus type 5 injection (H101) is the only approved oncolytic virus drug in China, primarily used for treating unresectable or recurrent nasopharyngeal carcinoma. AAV vector OBP-301, administered via intratumoral injection in combination with immunotherapy for esophageal cancer, has entered Phase I clinical trials. Additionally, the AAV vector AdVince, used for intratumoral treatment of neuroendocrine tumors, is currently in Phase I/IIa clinical trials.
AAV Neutralizing Antibodies (NAbs)
In AAV-based gene therapy, anti-AAV neutralizing antibodies (NAbs) bind to AAV, interfering with the virus's ability to attach to target cell surface receptors. This significantly reduces the transduction efficiency and effectiveness of the vector, directly impacting the therapeutic outcome. Furthermore, immune complexes formed by AAV and NAbs may trigger a series of immune responses, compromising the safety of gene therapy.
NAbs can arise from natural AAV infections or be induced following AAV vector administration. Pre-existing AAV antibodies in patients may result from natural infections, immune memory, or cross-reactivity, with approximately 30-60% of the population harboring pre-existing NAbs. Additionally, high-dose or systemic administration of AAV vectors may provoke host immune responses, leading to NAb production.
Screening for AAV NAbs is a critical step in gene therapy. Current methods for detecting AAV NAbs include cell-based assays and ligand-binding assays (e.g., indirect ELISA or chemiluminescence). These techniques are essential for detecting pre-existing antibodies, conducting neutralization assays, and measuring total antibody levels, all of which are crucial for ensuring the efficacy and safety of gene therapy.
Several regulatory guidelines now include AAV antibody screening as part of companion diagnostic testing. The approval of companion diagnostics (CDx) should align with the biologics license application for AAV-based gene therapies. AAV antibody testing is vital in systemic gene therapy, as it not only influences therapeutic outcomes but also ensures patient safety.
S-RMab® Monoclonal Antibodies
Starter has developed AAV (intact particle) antibodies that recognize specific conformational epitopes on AAV viral particles. These antibodies are used for quantifying viral physical titers and analyzing viral immunogenicity (anti-drug antibodies, ADA). In immunogenicity analysis, Starter's AAV antibodies can detect anti-capsid protein antibodies and neutralizing antibodies, serving as positive controls or for generating standard curves in ADA assays.
Product Name: AAV2 (intact particle) Recombinant Mouse mAb (S-R435) Catalog Number: S0B0771

AAV2 (intact particle) Recombinant Mouse mAb (S-R435) to be tested were diluted a series of gradients (from 500ng/ml, 2 fold, 9 points) ,and each gradient was co-incubated with AAV2 virus, the mixture was infected with 293T cells after incubation, and RLU luminescence value was detected.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B0771 | AAV2 (intact particle) Recombinant Mouse mAb (S-R435) | Host : Mouse | Inquiry |
| Conjugation : Unconjugated |




