Impact Factor: 27.4! START Secondary Antibodies Facilitate High-Efficiency RNA Delivery Research!


Since the COVID-19 pandemic, lipid nanoparticles (LNPs) for RNA delivery have become a highly popular research direction. In early February 2025, a team led by Yuefei Wang from Tianjin University published a study titled "Modular Design of Lipopeptide-Based Organ-Specific Targeting (POST) Lipid Nanoparticles for Highly Efficient RNA Delivery" in the journal Advanced Materials. The study focused on the modular design of lipopeptides (LPs) to develop lipid nanoparticles (LNPs) with high-efficiency RNA delivery capabilities and organ-specific targeting, named POST (Organ-Specific Targeting) LNPs.
1. Research Background
RNA therapeutics have shown great potential in treating various diseases, including cancer, infectious diseases, and genetic disorders. Lipid nanoparticles (LNPs) are one of the most promising RNA delivery systems, typically composed of ionizable lipids, helper lipids, cholesterol, and PEGylated lipids. However, a key challenge in LNP applications is their systemic liver accumulation in vivo, which limits their targeting ability to extrahepatic organs. Recent advancements include screening ionizable lipid structures, adding active targeting ligands, and modulating helper lipid or cholesterol properties. For example, the introduction of a fifth selective organ-targeting (SORT) helper lipid has enabled precise mRNA delivery to the spleen, lungs, and bone marrow.
2. Research Objectives
This study aimed to develop highly efficient ionizable lipids through the modular design of lipopeptides (LPs) to construct LNPs with organ-specific targeting capabilities. Lipopeptides combine the biocompatibility, designability, and targeting properties of peptides with the hydrophobicity of lipids, potentially enhancing the RNA delivery efficiency and targeting specificity of LNPs.
3. Research Methods
Design and Synthesis of Lipopeptides (LPs)
The researchers designed and synthesized a series of lysine-histidine-based lipopeptides (KH-LPs). Their structure includes a polar head (KHn, where n is the number of amino acids, 2-12) and a nonpolar tail (Ax or Ex, where A represents alkanol, E represents alkenol, and x is the number of carbon atoms, 10-18). Through a simple one-step ring-opening reaction, 25 different KH-LPs were synthesized and combined with four helper lipids (DOTAP, DOPE, DSPC, and 14PA) to construct a screening library of 100 four-component LP-LNPs.
Screening Strategy
The researchers evaluated the mRNA and siRNA delivery capabilities of these KH-LP LNPs through in vitro and in vivo screening. In vitro screening used human lung cancer cells (A549) as test cells, with mRNA expression efficiency and siRNA silencing efficiency measured by flow cytometry. In vivo screening involved injecting C57BL/6 mice with LNPs encapsulating luciferase mRNA and observing bioluminescence signals in different organs to assess organ targeting and delivery efficiency.
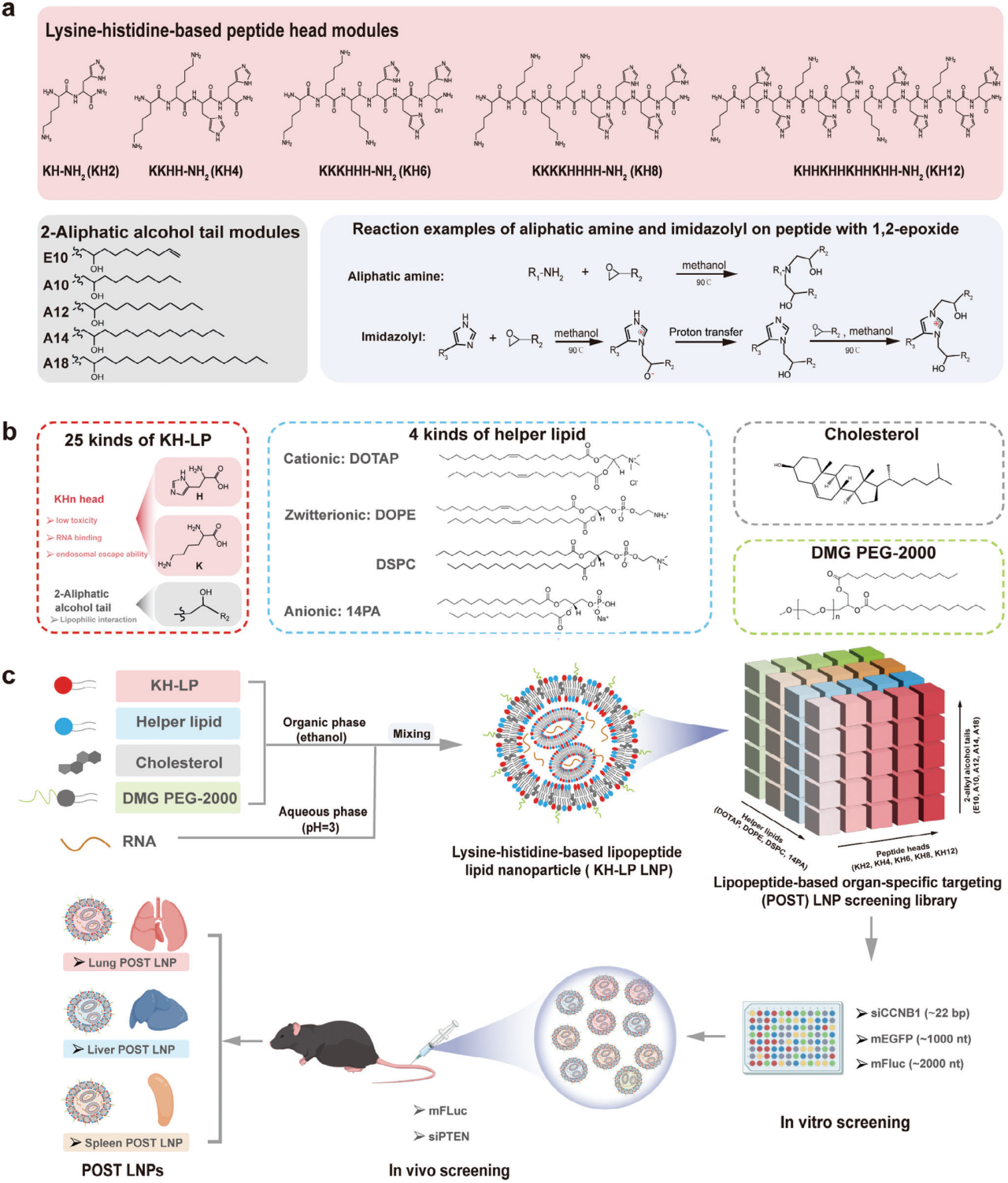
Modular Design of Lysine-Histidine-Based Lipopeptide Library (KH-LP) and Four-Component Lipopeptide-Based Organ-Specific Targeting (POST) LNP Screening Library
4. Experimental Results
In Vitro Screening Results
Among the 95 KH-LP LNPs with low cytotoxicity (cell viability >80%), 16 achieved over 70% mRNA expression efficiency in A549 cells, with A10-KH2/DOTAP performing best at 96.8%.
For siRNA delivery, 29 KH-LP LNPs achieved less than 20% relative target gene mRNA expression, with A10-KH2/DOTAP showing the highest silencing efficiency at 97.3%.
Further tests demonstrated that A10-KH2/DOTAP exhibited superior mRNA and siRNA delivery capabilities in multiple hard-to-transfect cell lines, outperforming commercial transfection reagents and the classic SM-102 LNP.
Structure-Activity Relationship (SAR) Analysis
Efficient KH-LP LNPs typically exhibited the following characteristics: pKa values between 6-7 (favorable for RNA delivery), encapsulation efficiency (ee) >80%, particle size <120 nm, polydispersity index (PDI) <0.2, and near-neutral zeta potential under neutral conditions.
Different helper lipid systems showed selectivity for KH-LPs. For example, DOTAP (permanent positive charge) was more suitable for short-chain KHn heads, while DOPE, DSPC, and 14PA (weak RNA binding capacity) were better suited for long-chain KHn heads to achieve efficient RNA binding, cellular uptake, and endosomal escape.
In Vivo Screening Results
In vivo experiments showed that A10-KH2/DOTAP exhibited lung-specific targeting, with bioluminescence signal intensity higher than that of classic lung-targeting LNPs (e.g., SORT MC3/DOTAP LNP).
A10-KH4/DOPE and A18-KH4/DOPE demonstrated efficient delivery to the liver and spleen, respectively, performing comparably or better than classic liver-targeting and spleen-targeting LNPs.
Cryogenic transmission electron microscopy (cryo-TEM) revealed that POST LNPs were spherical, with multilayered shells and an amorphous core.
siRNA Delivery Capability
In vivo siRNA delivery experiments showed that POST LNPs exhibited higher specificity and silencing efficiency in target organs compared to corresponding SORT LNPs.
In dose-dependent experiments, lung and liver POST LNPs achieved over 50% silencing efficiency at a dose of 0.1 mg/kg, while spleen POST LNPs achieved over 50% silencing efficiency at a dose of 0.01 mg/kg.
Stability and Biocompatibility
POST LNPs maintained stable mRNA delivery efficiency, particle size, PDI, and encapsulation efficiency after storage at 4°C for 9 days.
In repeated dosing experiments, mice showed sustained low levels of PTEN gene expression in target organs and an upward trend in body weight, indicating good biocompatibility of POST LNPs.
5. Conclusions
This study successfully developed a series of POST LNPs with high-efficiency RNA delivery and organ-specific targeting capabilities through the modular design of KH-LPs and helper lipids. These LNPs demonstrated excellent performance in both in vitro and in vivo experiments, with simple composition, good stability, and biocompatibility, highlighting their significant clinical potential. Additionally, SAR analysis indicated that precise modulation of LP structure and function can optimize ionizable components for different targeting lipid systems, providing new strategies for future RNA therapeutics.
This research not only offers new insights into the design of RNA delivery systems but also provides a theoretical foundation for developing organ-specific targeting nanomedicines. Through modular design and screening strategies, personalized RNA delivery systems can be developed for various diseases and target organs, potentially advancing the clinical application of RNA therapeutics.
S-RMab® Monoclonal Antibodies
The study utilized STARTER's fluorescence-labeled conjugated antibodies, Goat anti-Rabbit IgG (H+L) (Alexa Fluor® 594 Conjugate) (Catalog Number: S0B4007) and Goat anti-Mouse IgG H&L (Alexa Fluor 488 Conjugate) (Catalog Number: S0B4017), for immunofluorescence experiments. The experimental procedure was as follows:
1.POST LNPs loaded with mEGFP were intravenously injected into C57BL/6 mice (dose: 0.75 mg/kg).
2.After 48 hours, target tissues (liver, spleen, lungs) were isolated, and frozen sections (8 μm) were prepared and fixed with 95% ethanol for 10 minutes.
3.Sections were washed three times with PBS, permeabilized with 0.1% Triton X-100 for 30 minutes, and blocked with 5% normal goat serum for 1 hour.
4.Primary antibodies were incubated overnight at 4°C.
5.Slides were washed, and START secondary antibodies (1:500 dilution) were incubated at room temperature for 1 hour.
6.After washing, slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes and mounted.
7.Imaging was performed using an Olympus IX73 microscope.
8.PBS (1x) was used as a negative control.




