EGFR Family: How the "Conductors" of Cell Growth Become the "Accomplices" of Cancer? A Comprehensive Analysis from Targeted Therapy to Overcoming Drug Resistance

EGFR Family: How the "Conductors" of Cell Growth Become the "Accomplices" of Cancer? A Comprehensive Analysis from Targeted Therapy to Overcoming Drug Resistance
In the intricate regulatory network of cells, the epidermal growth factor receptor (EGFR) family acts as a group of "conductors" governing growth and differentiation. By precisely receiving external signals, they regulate cell proliferation, apoptosis, and migration. However, when these "conductors" go awry due to gene mutations, amplifications, or other reasons, they transform into "accomplices" fueling the uncontrolled growth of cancer cells. As a core target in cancer therapy, the EGFR family's research journey has not only unveiled key mechanisms of tumorigenesis but also driven revolutionary advancements, from monoclonal antibodies to small-molecule targeted drugs.
I. EGFR Family: The Central Hub of Signal Transduction and Structural Code
The EGFR family comprises four members: EGFR (HER1), HER2, HER3, and HER4. As transmembrane tyrosine kinase receptors, they form the control center of cell growth through a three-tiered mechanism: "extracellular domain recognizes signals - transmembrane domain transmits signals - intracellular domain activates pathways."
Structural Specificity Determines Functional Differences: HER2 lacks natural ligands but readily forms potent heterodimers; HER3 has weak kinase activity but serves as a signaling hub; HER4 can generate multiple isoforms through cleavage. These characteristics endow family members with distinct ligand binding, dimerization modes, and downstream pathway activation.
Signal Cascade Reactions: Ligand binding (e.g., EGF, TGF-α) triggers receptor dimerization, activating RAS-RAF-MAPK (proliferation), PI3K-AKT-mTOR (anti-apoptosis), and JAK-STAT (transcriptional regulation) pathways, ultimately influencing key gene expression such as CYCLIN D1 and BCL-2.
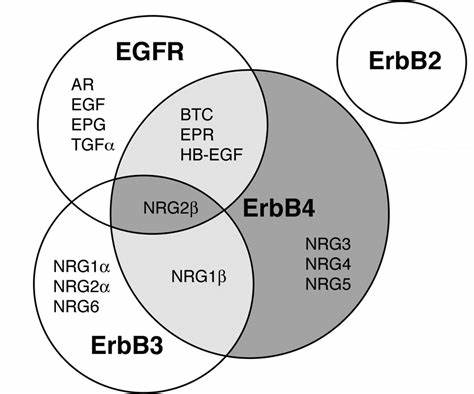
II. From Physiological Regulation to Oncogenic Dysregulation: The Dual Face of the EGFR Family
In normal tissues, the EGFR family safeguards development and homeostasis, but in tumors, it "rebels" through multiple mechanisms:
(I) Oncogenic Driving Mechanisms
Genetic Abnormalities: EGFR exon 19 deletions/L858R mutations in NSCLC lead to sustained kinase activation; HER2 gene amplification in breast cancer results in overexpression and formation of hyperactive signaling complexes.
Epigenetic and Microenvironmental Synergy: HER3 methylation causes dysfunction; ligand overexpression (e.g., EGF enrichment in the tumor microenvironment) triggers persistent receptor activation, promoting angiogenesis and invasion/metastasis.
(II) Cancer Cells' "Survival Package"
EGFR pathway dysregulation provides cancer cells with three "survival weapons": a proliferation engine accelerating G1/S phase transition, an anti-apoptotic shield inhibiting mitochondrial apoptosis pathways, and metastasis assistance promoting matrix degradation and angiogenesis. These changes collectively form the molecular basis of tumor malignancy.
III. Targeting the EGFR Family: From "Precision Strikes" to "Overcoming Drug Resistance"
Targeted drugs against the EGFR family employ dual strategies—"extracellular blockade" and "intracellular inhibition"—but face formidable challenges in drug resistance:
(I) Two Fronts of Therapeutic Arsenal
Extracellular Snipers—Monoclonal Antibodies
Herceptin (HER2): Blocks heterodimerization by binding to domain IV, revolutionizing HER2-positive breast cancer treatment.
Cetuximab (EGFR): Inhibits ligand binding and, when combined with chemotherapy, becomes a first-line regimen for KRAS wild-type colorectal cancer.
Intracellular Inhibitors—Small-Molecule TKIs
Gefitinib/Erlotinib: Competitively bind to EGFR kinase ATP sites, achieving an 80% objective response rate in EGFR-sensitive mutant NSCLC.
Lapatinib: Dual-targets HER2/EGFR, used as salvage therapy for Herceptin-resistant breast cancer.
(II) Drug Resistance: Cancer Cells' "Escape Strategies"
Target Mutations: EGFR T790M mutation increases ATP affinity, reducing TKI binding; HER2 L755S mutation weakens Lapatinib inhibition.
Pathway Compensation: KRAS G12V mutation activates downstream MAPK pathway "bypasses"; MET amplification forms a resistance network by cross-activating PI3K-AKT pathway.
Microenvironment and Stem Cells: Tumor microenvironment's low pH hinders drug penetration; cancer stem cells evade targeted killing through self-renewal.
IV. Breakthrough Strategies: Multidimensional Approaches to Drug Resistance
(I) Combination Therapy: Weaving a Signal Blockade Network
Dual-Target Synergy: Herceptin + Pertuzumab dual-target HER2 domains, reducing dimerization escape; EGFR-TKI + MET inhibitor counters MET amplification resistance.
Cross-Pathway Inhibition: BRAF inhibitor (e.g., Vemurafenib) + MEK inhibitor blocks KRAS-mutant tumor bypass activation.
(II) Drug Development: Breaking Traditional Target Limitations
Third-Generation TKIs: Osimertinib covalently binds EGFR T790M mutation sites, becoming standard therapy for NSCLC resistance.
Antibody-Drug Conjugates (ADCs): DS-8201 targets HER2 and releases cytotoxins, demonstrating breakthrough efficacy in HER2-low breast cancer.
(III) Precision Medicine: Biomarker-Guided Individualized Strategies
Dynamic Monitoring: ctDNA-based EGFR mutation profiling for real-time treatment adjustment; HER2 FISH testing to select optimal responder populations.
Nanotechnology Empowerment: Targeted delivery systems (e.g., folate-modified nanoparticles) enhance tumor drug enrichment and reduce normal tissue toxicity.
Conclusion: The Future Vision from "Target Discovery" to "Precision Control"
The EGFR family's research history is a "precision medicine evolution" from basic molecular mechanisms to clinical translation. Despite drug resistance as a current bottleneck, its complex regulatory network offers rich targets for drug development—from HER3 antibody MM-121 to bispecific antibody MM-151, from epigenetic regulation to tumor microenvironment intervention, multidisciplinary collaborations are spawning innovative therapies. In the future, with the popularization of liquid biopsy and AI-assisted drug design, EGFR family-related tumor treatment will advance toward an era of "more precise subtyping, predictable resistance, and personalized regimens," gradually realizing the medical vision of "targeting cancer cells while sparing normal ones."
In this decades-long "war on cancer," the EGFR family is both a formidable enemy and a pivotal breakthrough point. As research deepens, humanity will ultimately decipher the "dysregulation codes" of these "signal conductors," paving a broader path toward precision cancer therapy.
| Product Information | ||||
| Catalog Number | Product Name | Species | Conjugation | Price |
| S0B0878 | Histone H4 (acetyl K5 + K8 + K12 + K16) Recombinant Rabbit mAb (S-902-37) | Rabbit | Unconjugated | Inquiry |
| S0B0766 | Histone H3 (mono methyl K79) Recombinant Rabbit mAb (S-R417) | Rabbit | Unconjugated | Inquiry |
| S0B0546 | Histone H3 (acetyl K27) Recombinant Rabbit mAb (S-699-50) | Rabbit | Unconjugated | Inquiry |
| S0B0545 | Histone H3 (acetyl K4 + K9 + K18) Rabbit polyclonal antibody | Rabbit | Unconjugated | Inquiry |
| S0B0512 | Histone H3 (acetyl K9) Rabbit polyclonal antibody | Rabbit | Unconjugated | Inquiry |
| S0B0452 | Histone H3 (acetyl K27) Rabbit pAb | Rabbit | Unconjugated | Inquiry |
| S0B3393 | Amyloid beta 1-40 Recombinant Rabbit mAb (SDT-1548-54) | Rabbit | Unconjugated | Inquiry |
| S0B3398 | Amyloid beta (N-terminal) Mouse mAb (SDT-R457) | Mouse | Unconjugated | Inquiry |
| S0B3397 | Amyloid beta 1-42 Recombinant Rabbit mAb (SDT-1549-130) | Rabbit | Unconjugated | Inquiry |
| S0B1839 | Anti-EGFR Monoclonal Antibody (Cetuximab) (FITC Conjugate) | Human | FITC | Inquiry |
| S0B1413 | Phospho-EGFR (Tyr1068) Recombinant Rabbit mAb (S-1189-101) | Rabbit | Unconjugated | Inquiry |
| S0B1455 | Phospho-EGFR (Tyr1173) Recombinant Rabbit mAb (S-1187-106) | Rabbit | Unconjugated | Inquiry |
| S0B1460 | Phospho-EGFR (Tyr1173) Recombinant Rabbit mAb (S-1187-135) | Rabbit | Unconjugated | Inquiry |
| S0M1046 | EGFR Signaling MiniAb Kit (Human) | $2,100 | ||
| S0M1047 | EGFR Signaling MiniAb Kit | $1,380 | ||
| S0B2355 | EGFR (L858R) Recombinant Rabbit mAb (SDT-421-202) | Rabbit | Unconjugated | Inquiry |
| S0B2355P | EGFR (L858R) Recombinant Rabbit mAb, PBS Only (SDT-421-202) | Rabbit | Unconjugated | Inquiry |
| S0B1478 | Phospho-EGFR (Tyr1068) Rabbit Polyclonal Antibody | Rabbit | Unconjugated | Inquiry |
| S0B1482 | EGFR Rabbit Polyclonal Antibody | Rabbit | Unconjugated | Inquiry |




