Copper-Induced Cell Death: The New Frontier in Cancer Research

Background Introduction
Since the team of Peter Tsvetkov and Todd R. Golub from the Broad Institute of MIT and Harvard University published the paper “Copper induces cell death by targeting lipoylated TCA cycle proteins” in Science in 2022, a novel form of cell death caused by copper — copper-induced cell death (cuproptosis) has gradually become a research hotspot among scientists.
Copper (Cu) is an essential micronutrient that plays a crucial role in promoting metabolism and maintaining various fundamental biological functions. The homeostasis of copper in the human body is strictly regulated, and the imbalance of copper homeostasis can lead to metabolic abnormalities and exert toxic effects on cells. Cuproptosis is a newly discovered form of programmed cell death caused by excessive intracellular copper, which is distinct from other known cell death pathways (such as apoptosis, necrosis, pyroptosis, and ferroptosis), and it shows great potential in cancer therapy.
Copper Metabolism
In biological systems, copper typically exists in two oxidation states, Cu+ and Cu2+, and its biological functions are closely related to its ability to cycle between oxidized and reduced forms. Copper is mainly obtained from organ meats and shellfish, and the recommended daily copper intake for adults is 0.8–2.4 mg to maintain systemic copper homeostasis. However, excessive or insufficient levels of copper can lead to cytotoxicity and pathological changes. Therefore, it is essential to maintain systemic copper levels within a certain range.
The absorption of dietary copper primarily occurs in the duodenum and small intestine, and the uptake of copper by enterocytes relies on copper transporter 1 (CTR1). Prostate stem cell antigen can act as a copper reductase to maintain the reduced state of copper, thereby promoting CTR1-dependent copper uptake. Mediated by ATP7A, copper is transported into the bloodstream and binds to copper chaperone proteins (such as ceruloplasmin, serum albumin, histidine, etc.). Copper is transported to the liver via the portal vein system (the liver is the central control system for copper homeostasis and is also the main organ for copper storage and excretion), where it is chelated by metallothionein 1 and 2 for storage in hepatocytes. Mediated by ATP7B, copper is released into the bloodstream, binds again to soluble chaperone proteins, and is transported to specific tissues and organs. Upon reaching its target tissues, copper catalyzes reactions in various physiological processes, including mitochondrial energy production, tyrosine and neurotransmitter metabolism, redox homeostasis, and extracellular matrix remodeling. Excess copper is excreted through bile (the primary form of endogenous copper elimination) or is excreted from the body as unabsorbed metal ions via feces. Therefore, the status of copper in the body is dynamically regulated by intestinal absorption, hepatic storage, and biliary excretion.
In the cytoplasm, the distribution of copper and its biological functions are mainly regulated through five pathways coordinated by copper chaperone proteins:
a. Copper is transferred to metallothionein via glutathione (GSH);
b. Antioxidant 1 copper chaperone protein transports copper to ATP7A and ATP7B in the trans-Golgi network to regulate copper homeostasis;
c. Copper is transported to the nucleus, where the zinc finger structure of Sp1 acts as a copper sensor to regulate the expression of CTR1;
d. Superoxide dismutase copper chaperone protein transports copper to superoxide dismutase 1 to scavenge free radicals;
e. Cytochrome c oxidase copper chaperone protein transports copper from the cytoplasm to the mitochondria, participating in oxidative phosphorylation and maintenance of mitochondrial function. When copper homeostasis is disrupted, severe diseases can occur, such as Menkes disease and Wilson disease. Moreover, copper deficiency is also present in diseases like Alzheimer’s disease, Parkinson’s disease, non-alcoholic fatty liver disease, diabetes, and obesity.
The Relationship Between Copper Levels and Cancer
Studies have shown that the levels of copper in the tumors and serum of patients with breast cancer, lung cancer, gastric cancer, thyroid cancer, and prostate cancer are significantly elevated[2]. Copper affects tumor occurrence, angiogenesis, tumor recurrence, and metastasis by binding to and activating key molecules in multiple signaling pathways.
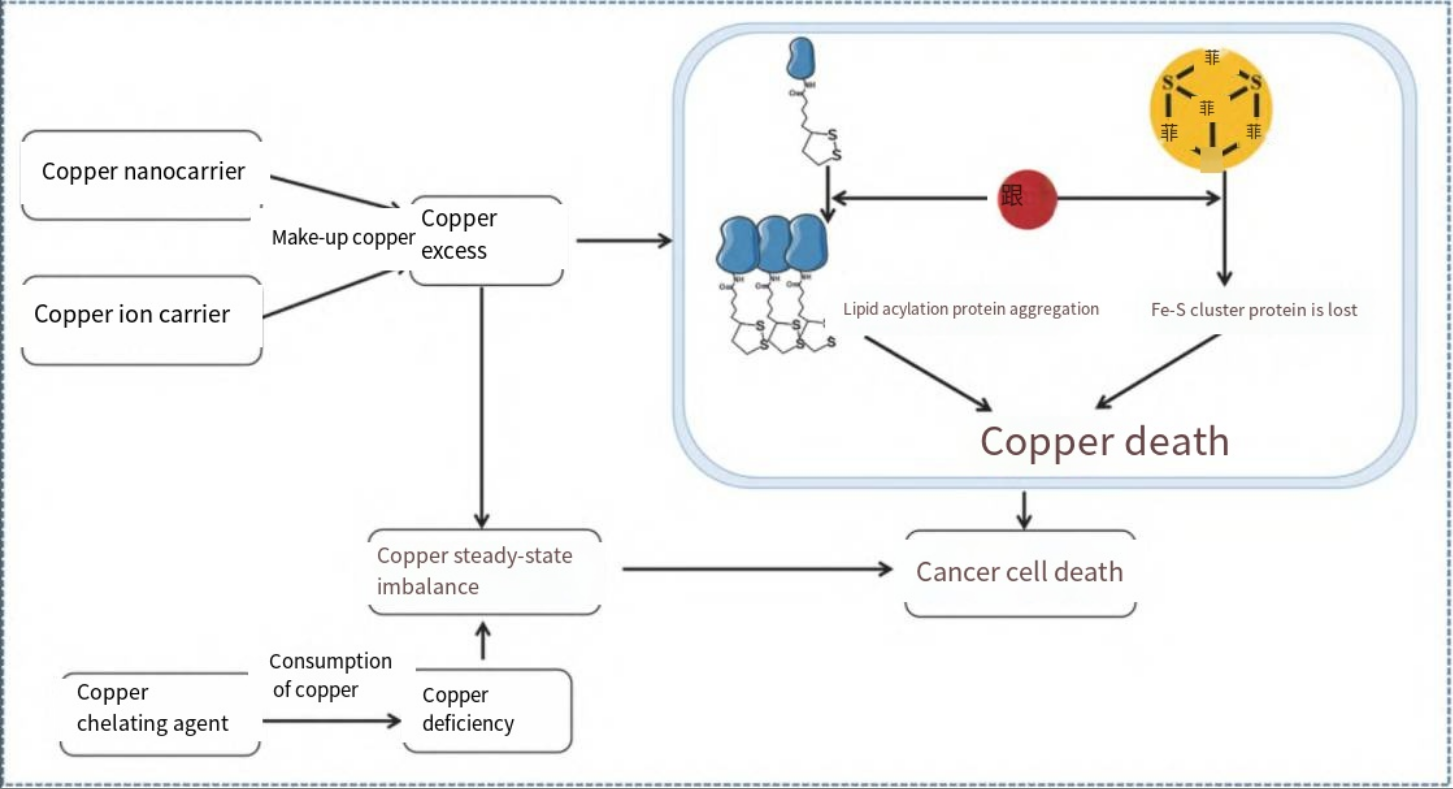
Figure 1 The relationship between copper levels and cancer[1]
Copper can act on receptor tyrosine kinase-related signaling pathways, phosphoinositide-3-kinase signaling pathways, and mitogen-activated protein kinase signaling pathways to promote cell growth and proliferation. In addition, copper, as a “switch” angiogenic messenger, can activate angiogenic factors such as tumor necrosis factor, stimulate the proliferation of vascular endothelial cells, stabilize hypoxia-inducible factor-1, and promote inflammatory angiogenesis. Copper can also promote tumor cell invasion and migration by activating enzymes and signaling cascades related to metastasis. Moreover, copper can regulate phosphodiesterase 3B to modulate tumor metabolism. Therefore, copper can serve as a key target for anti-tumor therapy.
The Application of Copper Ion Detection in Gastric Cancer Therapy
On October 20, 2023, the research team led by Professor Huang Chen and Professor Fan Guangjian from the First People’s Hospital of Shanghai published a research paper titled “Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer” in Nature Communications. The study revealed the molecular mechanism by which METTL16 regulates cuproptosis in gastric cancer cells, clarified the significant importance of lactylation and m6A modification in the progression of gastric cancer, and provided new targets and strategies for the treatment of gastric cancer.
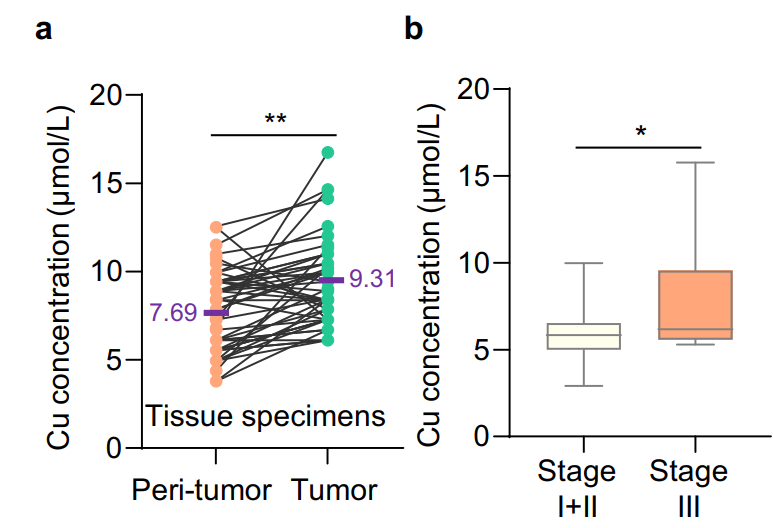
Figure 2 Higher copper content in gastric cancer tissues is associated with tumor progression[3].
a. The concentration of copper in gastric cancer tissues (green dots) (n=48) was significantly higher than that in adjacent normal tissues (orange dots) (n=48), (purple font indicates the mean copper concentration, P<0.01).
b. The relative copper content in patients with stage III gastric cancer was higher than that in patients with stage I and II gastric cancer (P<0.05).
Operation Steps of Copper Ion Detection Kit
I. Kit Components
|
Component |
Specification |
Storage Conditions |
|
96-Well Microplate |
1 plate |
— |
|
Assay Buffer |
30mL × 4 |
4 ℃ |
|
Reaction Buffer |
4mL × 1 |
4 ℃ |
|
Masking Reagent |
Powder × 1 |
4 ℃ |
|
Dye Reagent |
Powder × 1 |
4 ℃, protected from light |
|
Dye Reagent Diluent |
1mL × 1 |
4 ℃ |
|
Standard (250μmol/L) |
1mL × 1 |
4 ℃ |
|
Technical Manual |
1 Manual |
— |
II. Operation Steps:
1. Materials and Reagents:
Materials to be provided: Microplate reader, distilled water, pipette and tips, centrifuge, timer, wet ice, etc.
Standard: Dilute the standard with distilled water in a two-fold gradient (prepare fresh for use).
Masking Reagent: Dissolve with 1mL distilled water (prepare fresh for use).
Dye Reagent: Dissolve the Dye Reagent with 1mL Dye Reagent Diluent, heat to assist dissolution at 70℃ (prepare fresh for use).
2. Sample Processing:
a. Tissue samples: Weigh 0.1g of tissue, homogenize in 1mL Assay buffer on wet ice, centrifuge at 4℃, 10000g for 10min, transfer the supernatant to a new centrifuge tube, and store on wet ice for detection.
b. Cell/bacterial samples: Collect cells/bacteria (5×106) into a centrifuge tube, remove the supernatant after centrifugation, add 1mL Assay buffer, sonicate (power 20%, sonicate for 3s, pause for 10s, repeat 30 times), then centrifuge at 4℃, 10000g for 10min, transfer the supernatant to a new centrifuge tube, and store on wet ice for detection.
c. Liquid samples: Detect directly.
3. Loading: Add 140uL of standards and samples of different concentrations to the corresponding wells, respectively, and set a blank well with 140uL distilled water; add 40uL Reaction Buffer, 10uL Masking Reagent, and 10uL Dye Reagent to each well, mix well, and incubate at room temperature for 15min.
4. Detection: Measure the absorbance at 605nm using a microplate reader, fit the standard curve, and calculate the sample concentration.
Frequently Asked Questions
Q1: Does the kit have any requirements for the processing of tissue samples?
A1: Metal chelators (such as EDTA) will interfere with this analysis and should be avoided in sample preparation.
Q2: Does the kit detect Cu+ or Cu2+ or total copper?
A2: The kit detects total copper.
Q3: Is there a recommended dilution factor for samples?
A3: For unknown samples, we recommend performing a pre-experiment with several dilution gradients to determine the appropriate dilution factor.
Q4: The kit instruction manual includes both standard curve method and formula method. Which calculation method should I choose?
A4: Either method is fine. The formula is a simplified calculation. It is recommended to calculate according to the standard curve, which will be more accurate. It should be noted that when using the standard curve method, a new standard curve must be drawn for each experiment.
Q5: How should the Dye Reagent in the kit be stored after dilution and dissolution?
A5: The dye should be prepared fresh for use. After dissolution, it is prone to oxidation and deterioration. It is recommended to aliquot and store at 4℃, protected from light.
References
[1] Shi X, Du X. Mechanisms of copper-induced cell death and related anti-cancer drugs[J]. Chemistry of Life, 2024, 44(2): 225-232.
[2] Xie J, Yang Y, Gao Y, et al. Cuproptosis: mechanisms and links with cancers[J]. Mol Cancer, 2023, 22(1): 46.
[3] Sun L, Zhang Y, Yang B, et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer[J]. Nature Communications, 2023, 14(1).
Recommended Biochemical Detection Kits:
|
Catalog Number |
Product Name |
Specification |
Detection Range |
|
Copper Ion Detection Kit |
96T |
0.625 μmol/mL - 20 μmol/mL |
|
|
Creatinine Detection Kit |
96T |
1 μg/mL - 100 μg/mL |
|
|
Blood Urea Nitrogen (BUN) Detection Kit |
96T |
1 μmol/L - 250 μmol/L |
|
|
Malondialdehyde (MDA) Detection Kit |
96T |
0.01 mmol/L - 1 mmol/L |
|
|
Taurine Detection Kit |
96T |
0.05 mmol/L - 1 mmol/L |
|
|
Glycerol Detection Kit |
96T |
0.05 mmol/L - 5 mmol/L |
|
|
Glutamine Detection Kit |
96T |
0.1 mmol/L - 10 mmol/L |
|
|
Lactate Dehydrogenase Detection Kit |
96T |
0.1 mmol/L - 10 mmol/L |
|
|
Tryptophan Detection Kit |
96T |
0.05 mmol/L - 10 mmol/L |
|
|
Ammonia Detection Kit |
96T |
1 μmol/L - 1000 μmol/L |
|
|
Hydroxyproline Detection Kit |
96T |
2 μmol/L - 200 μmol/L |
|
|
Glucose Detection Kit |
96T |
0.1 mmol/L - 10 mmol/L |
AntBio provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.




