Cellular ATP luminescence detection strategy

Detection principle
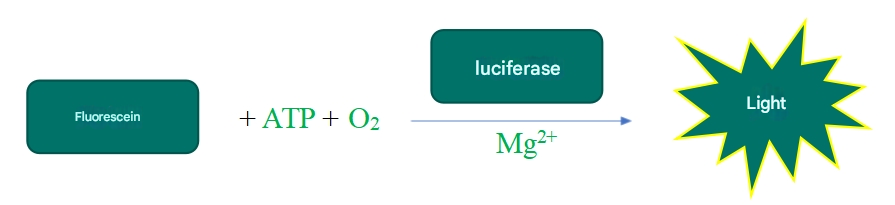
The 2D Luminescent Cell Viability Assay uses an ATP-dependent luciferase-catalyzed fluorescein luminescence reaction to determine the intracellular ATP content through chemiluminescence signal, thereby detecting cell viability or quantitatively detecting the number of viable cells, with a wide linear range, high sensitivity and good stability. There is a good linear relationship between 100 and 100,000 cells in a 96-well plate, but the upper limit of the assay will vary from cell to cell. In addition, the operation is simple, the assay reagent provided in the kit is ready-to-use, the readability is stable, the detection speed is fast, and the assay is completed in about 10 minutes, without the need to wash cells, and there is no need to change or remove the culture medium。 Compared with other common cell viability assays, such as Calcein-AT, CCK-8, etc., the 2D Luminescent Cell Viability Assay is simpler and faster.
Product Advantages:
The 2D Luminescent Cell Viability Assay (abs50065) has high sensitivity and a wide linear range, making it compatible with the detection of small sample volumes as well as high-throughput screening of large sample volumes.
1. Convenient and fast: The detection reagent provided in the kit is ready-to-use, with stable reading, fast detection speed, and only takes about 10 minutes to complete the test;
2. High sensitivity: it can detect ATP at a minimum of 10nmol;
3. Wide linear range: In the 96-well plate, there is a good linear relationship in the range of 100 to 100,000 cells, but the upper limit of the detection number of different cells will be different;
4. High-throughput: It is compatible with the detection of a small number of samples and the high-throughput screening of a large number of samples.
How to use:
1. Reagent preparation
Reagent melting: The reagent was removed the day before the experiment and placed at 4°C overnight to thaw. The reagent can also be removed on the day of the experiment and thawed at room temperature, or placed in a 22°C water bath to melt, but it should be noted that the water temperature should not exceed 25°C.
Equilibrate to room temperature: If the reagent melts at non-room temperature, place it in a 22°C water bath before use to ensure that the reagent is equilibrated to room temperature before testing.
Note: Generally, it takes about 10min for 5mL packaging; The 50mL package takes about 20 min.
Mix: Mix the solution by gently inverting 5 times before use.
Add Triton X-100 (abs9149): Aspirate the volume of reagent required for the experiment and add the appropriate volume of Triton X-100 to a final concentration of 0.2%. This liquid is the assay solution (e.g., 8 mLATP assay plus 0.2 mL Triton X-100).
Second, the detection steps
1. Cell culture
Seeding 100 μL of cells per well using a 96-well plate (ABS7149) suitable for chemiluminescent detection (determine the initial seeding cell density according to the culture time, the number of cells per well should not exceed 100,000 at the time of detection), and set up the well without the culture medium as a negative control. It is also possible to set the concentration gradient of the cells to get the best results. 37℃,5% CO2Culture the cells and treat them with dosing at the appropriate time as needed.
2. (Optional) Preparation of ATP standard curve
The prepared ATP standard solution is diluted with PBS into an appropriate concentration gradient and 100 μL of standard is added to each well of the 96-well plate.
3. Cell viability detection
(1) thaw the luminescence detection reagent frozen and equilibrate to room temperature (or equilibration in a constant temperature water bath at 22 °C), take out the volume of detection reagent for the experiment, and add an appropriate amount of Triton X-100 (so that its final concentration is 0.2%);
(2) Take out the cell culture plate and equilibrate it at room temperature for 10min (or equilibrate at 22°C, the time should not be too long, and try to control it within 30min);
(3) 100 μL of detection reagent is added to each well of a 96-well plate (due to the edge effect of the wells, the luminescence signal may be unstable, and it is not recommended to plate at the edges);
(4) Shake at room temperature for 2min to promote cell lysis;
(5) Place at room temperature for 10min to stabilize the luminescence signal;
(6) Chemiluminescence detection using a multifunctional microplate reader. Set the corresponding parameters according to the requirements of the instrument, and the detection time of each well is generally 0.25-1s, which needs to be adjusted appropriately according to the detection sensitivity of the instrument;
(7) Calculate the relative viability of the cells based on the chemiluminescence readings, or calculate the ATP content according to the ATP standard curve to obtain the relative viability of the cells.
Note: The results of the assay vary depending on the type of cell, but for some cells with particularly high ATP content, the chemiluminescence reading may continue to rise above 100,000 cells, but the linearity is lost.
3. Precautions
1. Temperature: The reagent contains luciferase, and repeated freezing and thawing will affect its activity. It is recommended to store at -20°C in the dark after aliquoting. Both reagents and cell samples need to be balanced to room temperature before use to avoid the influence of enzyme catalytic effect;
2. Chemical factors: The reaction rate and luminescence intensity of luciferase are affected by the chemical environment. There are differences in luminous intensity and decay rate in different types of media and serum. In addition, high drug levels may interfere with the luciferase reaction, which can affect the luminescence signal. It is recommended to set up control wells of cell culture medium containing the drug to rule out solvent interference. If the medium has a large effect on the luminous intensity, it can be removed before detection and rinsed with PBS once, at this time, the addition of Triton X-100 is halved to a final concentration of 0.1%;
3. Light sensitivity: This reagent is sensitive to light, and if it is exposed to light during storage, it will accelerate the attenuation of luminous intensity. If the reagent is transferred from the original container, make sure to store it away from light;
4. ATP contamination: It is recommended to wear masks and latex gloves during operation to avoid contact with potentially contaminated surfaces and equipment. Avoid inserting the tip tip into the reagent bottle multiple times when operating.
That's all for today's explanation, if you have any questions about cell experiments, welcome to join the group to communicate!
This issue is recommended by Xiaoai
|
Catalog number |
name of article |
specification |
|
2D Luminescent Cell Viability Assay |
50mL×2 |
|
|
abs50059 |
2D/3D/Organoid ATP Viability Assay Kit |
100mL |
|
abs9149 |
Triton X-100 |
500mL |
|
abs7149 |
Ultra-low adsorption plates (96-well plates, black framed clear bottom transparent lids) |
1 box |
|
abs7151 |
Ultra-low adsorption plates (96-well plates, white framed clear bottom clear lid) |
1 box |
|
abs7053 |
10 mL disposable pipettes |
1 box |
|
25 mL disposable pipette |
1 box |
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |




