Why are organoids not long after passage?

Why are organoids not long after passage?
I believe that everyone looks at this title, and their hearts are heavy, especially for some hard-to-raise organoids, which are finally raised, but they can't grow up after the generations, what is the situation? Today, let's analyze the reasons for the failure of organoid passage from the following three levels.
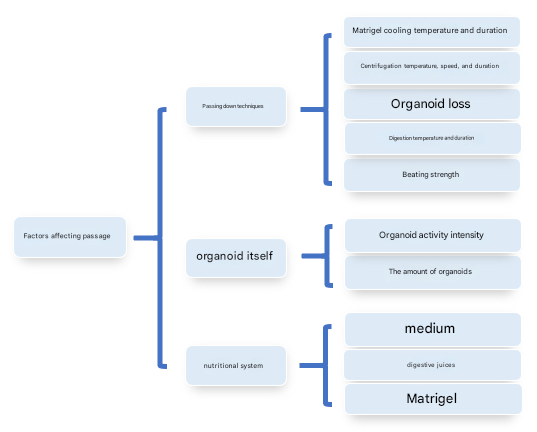
Factors influencing organoid passaging
1. Organoid passaging techniques
The most important factor influencing organoid passaging is the passaging technique. In particular, some delicate organoids, like flowers in a greenhouse, cannot withstand any "wind and rain", such as the matrigel cooling conditions (temperature and length), centrifugation conditions (temperature, speed, duration), the loss of organoids when discarding the supernatant, digestion conditions (temperature, duration), and blowing intensity are "ninety-nine eighty-one difficulties on the road to learning scriptures in the west".
(1) Matrigel cooling temperature and duration
For organoid passaging, we need to blow off the Matrigel and organoid suspension with 5-10 wells (24-well plates, for example) and collect them into a 15 mL centrifuge tube with organoid passaging buffer (abs9730), and use organoid passaging buffer to 14 mL. Next, you can choose a variety of ways to cool the matrix gel, such as pre-cooling at 4°C for 30min; Pre-cooling at 0°C for 30min; Pre-cool at -20°C for 5min. In general, most organoids can take all three approaches, but a few organoids are too sensitive to temperature or duration. For organoids that are too sensitive to temperature, it is recommended to pre-cool at 4°C for 30min or pre-chill at 0°C for 30min; For organoids that are too sensitive to time, it is recommended to pre-cool at -20°C for 5min.
(2) Centrifugation temperature, speed and duration
The possible damage caused by centrifugation to organoids mainly includes:
A. Mechanical stress damage: The mechanical stress generated during centrifugation may cause damage to organoid cells. This damage may include rupture of cell membranes, damage to the internal structures of cells, and loss of cell function.
B. Temperature influence: The temperature during centrifugation has an important impact on the preservation state of organoids. If the centrifugation temperature is too high, it may cause the Matrigel to solidify, affecting the isolation and preservation of organoids. Therefore, centrifugation at a low temperature of 4 °C is recommended to avoid thermal damage.
After the matrigel is cooled, it is recommended to use a horizontal angle centrifuge with a speed control of 200g-300g, a temperature of 4°C, and a time control of less than 5min.
(3) Loss of organoids when discarding the supernatant
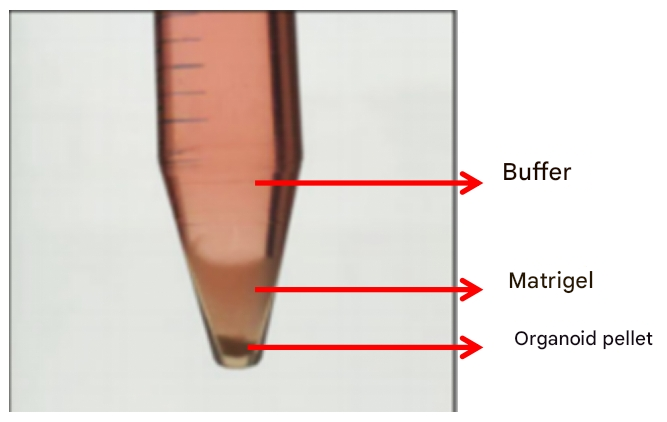
Stratification phenomena (buffer, Matrigel, organoid precipitation)
After centrifugation, the organoids are normally layered in three layers: buffer, Matrigel, and organoid precipitate, and normally we retain the organoid precipitate layer, and the buffer and Matrigel on it are removed. However, if the number of organoids cultured in our primary generation is very small, the sediment layer of organoids after stratification will be very small, in this case, we must not only retain the organoid precipitation, but also retain the lower 2/3 of the Matrigel, or even the full layer of Matrigel, to reduce the loss of organoids (the Matrigel layer will remain organoids). When dispensing, ensure that the amount of new glue is more than 1.5 times the volume of the residual glue to ensure the adhesion of the matrice.
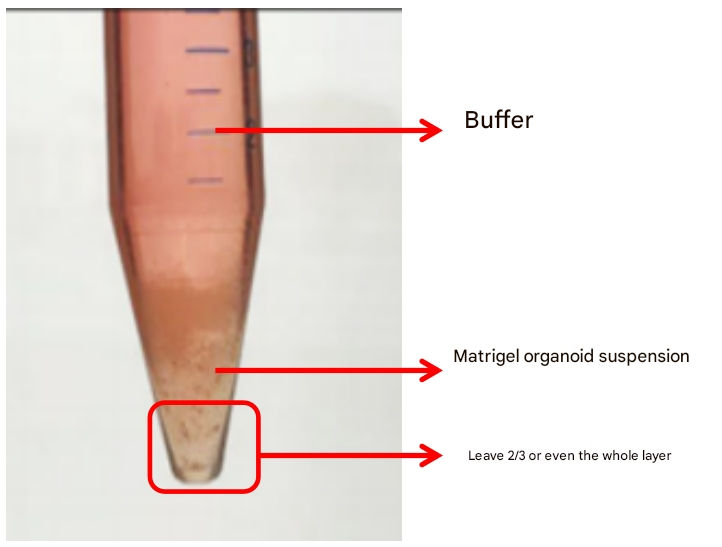
Of course, sometimes there are two layers of centrifuged organoids: buffer and Matrigel organoid suspension. In this case, it is recommended to keep 2/3 of the Matrigel organoid suspension, or even the full thickness of Matrigel, to reduce the loss of organoids (the Matrigel layer will remain organoids). When dispensing, ensure that the amount of new glue is more than 1.5 times the volume of the residual glue to ensure the adhesion of the matrice.
(4) Digestion temperature and duration
When the above-mentioned centrifuged organoid suspension is digested and passaged, special attention should be paid to the control of digestion temperature and duration. The damage to organoids caused by overdigestion is manifested in two ways:
A. Cell membrane damage and cell death: Excessive digestion may damage the cell membrane, resulting in the release of cell contents into the culture medium, causing cell death.
B. Increased apoptosis: Trypsinization for too long may increase the apoptosis rate of cells. This is because excessive digestion may cause damage to the cell membrane and affect the functionality and integrity of cell surface receptor proteins.
Although the activity of digestive enzymes is best at 37°C, it is not necessarily a good thing for organoids if the digestion is too strong. At the same time, the digestion time is recommended to be 2-3min (the longer the digestion time, the more damage caused by enzymes to organoids).
(5) Blowing strength
There are two main forces generated by tip blowing in organoid cultures: mechanical damage and shear forces.
A. Mechanical damage: This mainly refers to the cell damage caused by the scraping of the pipette tip and the collision between cells. In order to reduce this part of the damage, it is necessary to reduce the blowing force as much as possible, as long as it can be blown off and the blown away cells. Contact between the tip and the cell layer or even grinding can lead to direct cell death, so it is necessary to control the distance between the tip and the bottom of the plate to avoid direct contact.
B. Shear force: Shear force refers to the phenomenon that the cross-section of the object is affected by an external force and moves relative along the direction of the external force, and this external force is called shear force. In cell culture media, if there are a lot of air bubbles, the cells are between the bubbles and the medium, and when the bubbles are broken, the cells will be affected by strong shear forces instantly, resulting in cell fragmentation.
If mechanical pipetting passaging is chosen, the intensity and frequency of pipetting have a great impact on organoid activity. If you use a 1mL pipette tip to pipette, the operation must be gentle to avoid cutting caused by multiple organoids due to the shear force of the liquid flow, and you can also use a sterile Bass pipette instead of the pipette head, which will cause little damage to the organoids. At the same time, the number of pipetting is controlled within 30 times, which can be observed under the microscope at any time, and once the organoids are dissociated from the Matrigel and there are more cell clumps, the pipetting can be stopped. Therefore, special attention needs to be paid to the control of strength and technique when performing organoid pipetting to reduce the damage to the cells.
2. The organoids themselves

Lung cancer organoids do not grow long within a week after passage
(1) Organoid activity intensity
The activity of different types of organoids varies greatly, and the organoids with strong activity include endometrial cancer organoids and intestinal cancer organoids, which are relatively easy to culture, and can even reach more than 30 generations in a row. However, some organoids themselves are not active enough, such as thyroid cancer organoids, which can only maintain the primary generation, and basically do not last long after passage. In addition, organoids that are recognized as difficult to maintain include liver cancer organoids and prostate cancer organoids.
(2) Number of organoids
If the number of organoids in the primary culture is scarce, such as a 24-well plate with 50 uL of stromal glial cell mass suspension per well, and the number of grown organoids is less than 20, it is usually the case that the number of organoids is low. At this time, if the passage operation is improper, a large number of organoids will be lost, which will lead to the density of cell clusters in the passage being too thin, the lack of paracrine signal regulation, the difficulty of organoid proliferation, and the phenomenon of small growth. Therefore, the number of primary organoids also directly affects whether they can be passed smoothly.
3. Nutritional system
(1) Culture medium
Complete medium (added factors) is recommended to be stored at -20 °C in aliquots with an expiration date of up to 1 year; Store at 4°C, it is recommended to use it up within 2 weeks, after 1 month, the factor activity will gradually decrease.
Storing at 4°C for too long (more than a month) can lead to a decrease in the efficiency of the medium, but how does the decrease affect the passage of organoids?
For example, the same bottle of medium is used before and after digestion and passage, and the primary generation can be raised, but the passage is not. When you infer the reason, you may first rule out the problem of the medium, because the same bottle of medium is used before and after digestion and passage, if it is a problem with the medium, the primary generation is not good, and the result is that the primary generation is well raised and the passage is not good. In fact, there are factors that you can't think of here. As we all know, organoids usually slow down the proliferation of organoids (such as decreasing the number and shrinking size) after a certain generation, and they cannot be transmitted. Because adult stem cells or cancer stem cells cultured in organoids will have a decrease in stemness (self-renewal and differentiation ability) after multiple passages, and finally cannot be transmitted. In primary culture of organoids, tumor stem or adult stem cells are very dry and can proliferate even when stored at 4°C for too long in medium. However, when the stemness of tumor stem or adult stem cells gradually decreases after passage, the demand for factor activity of the medium will increase, and it will be difficult to expand the organoids by using the medium stored at 4°C for too long.
(2) Organoid passage digestive juices
The effect of digestive juices on organoid passage is mainly reflected in two aspects:
A. Whether the passage digestive juice is appropriate, and inappropriate enzymes can cause organoid damage.
B. It is recommended to store the passaging digest at -20 °C in separate packages, with a validity period of up to 1 year, and stored at 4 °C, it is recommended to use it up within 2 weeks, and the enzyme activity will gradually decline after 1 month.
First of all, the strength of digestive enzymes also depends on the type of enzyme. We have tried pancreatic enzymes for organoid digestion and found that pancreatic enzymes are relatively damaging to the digestion of organoids, and are not suitable as organoid subdigesting enzymes. Therefore, it is recommended to use a special organoid passage digest (ABS9520), which uses a variety of mild digestive enzymes to digest organoids. Secondly, if the digestive juice is stored at 4 °C for a long time, the enzyme activity will gradually decrease, and the time required to digest the organoids will want to increase, and the increase in digestion time and improper control of the time will easily lead to excessive digestion and damage the activity of the organoids.
(3) Matrigel
Matrigel is recommended to be stored at -20 °C in aliquots, with an expiration date of up to 1 year, stored at 4 °C, it is recommended to use it up within 2 weeks, and after 1 month, the adhesion and factor activity of Matrigel will gradually decrease.
Matrigel has two functions, one is to play the role of scaffolds to maintain the ellipsoid morphology of organoids, and the other is to contain rich factor species, which has a promoting effect on the growth of organoids. When the Matrigel is stored at 4°C for too long, the adhesion and factor activity of Matrigel will be greatly reduced, and the structure of the Matrigel organoid dome at this time is easy to float away from the bottom surface of the culture plate, and it is also difficult to maintain the demand for organoid proliferation.
That's all for today's explanation, if you have experimental questions, welcome to join the group to communicate!
Organoid culture product recommendation
|
Catalog number |
name of article |
specification |
|
abs9920 |
Organoid recovery solution |
100mL |
|
abs9730 |
Organoid subculture buffer |
250mL/500mL |
|
abs9520 |
Organoid passaging digestive juices |
100mL/500mL |
|
Matrigel (low factor, no phenol red) |
1.5mL×4/1.5mL×8 |
|
|
Organotial Human Bowel Cancer Organoid Culture Kit |
1kit |
|
|
Organotial Human Hepatic Cholangiocarcinoma Organoid Culture Kit |
1kit |
|
|
abs50059 |
2D/3D/Organoid ATP Viability Assay Kit |
10mL/100mL |




