UFMylation: A New Hub in Immunoregulation, Zhou Junzhi's Team Unveils Potential Therapeutic Targets for Disease Treatment

UFMylation: A New Hub in Immunoregulation, Zhou Junzhi's Team Unveils Potential Therapeutic Targets for Disease Treatment
In the mysterious realm of protein modification, a novel ubiquitin-like modification discovered in 2004—UFMylation—is gradually unveiling its pivotal role in immunoregulation. Recently, Zhou Junzhi's research group from the School of Basic Medical Sciences at Guangdong Medical University published a significant review in Clinical and Translational Medicine (Q1 in Chinese Academy of Sciences, IF=7.9), systematically elucidating how the UFMylation system acts as a central hub connecting cellular homeostasis and disease pathogenesis through precise regulation of immune responses, offering novel therapeutic strategies for tumors, infectious diseases, and more.

I. The UFMylation System: From Molecular Mechanisms to Life Regulatory Networks
As a highly conserved modification system in eukaryotes (excluding fungi such as yeast), the UFMylation system comprises UFM1 molecules, a three-tier enzymatic cascade (E1 activating enzyme UBA5, E2 conjugating enzyme UFC1, E3 ligase UFL1), de-UFMylation enzymes (UFSP1/2), and adaptor proteins (DDRGK1, C53). Its core function lies in the reversible lysine modification that precisely regulates target protein functions: mature UFM1 is covalently conjugated to substrates via UBA5/UFC1/UFL1 catalysis and removed by UFSP1/2 after fulfilling its role, forming a dynamic equilibrium. This process deeply participates in fundamental life activities such as endoplasmic reticulum (ER) homeostasis, genomic stability, and the cell cycle, with dysregulation closely associated with neurodevelopmental disorders such as developmental dysplasia of the hip, infantile encephalopathy, and microcephaly.
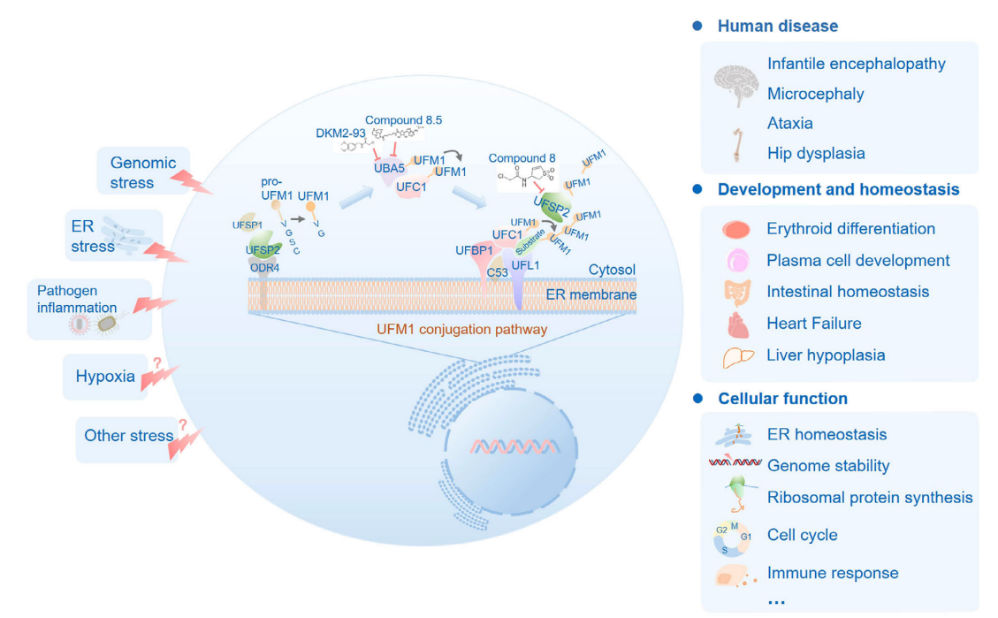
Composition and Function of UFMylation
II. Dual Faces of Immunoregulation: Precise Regulation of Innate and Adaptive Immunity
- Innate Immunity: The "Dual-Edged Sword" of Antiviral and Inflammatory Responses
In the antiviral defense line, the UFMylation system exhibits a "double-edged sword" effect: during HSV-1 infection, UFL1 binds to STING protein, inhibiting its ubiquitin-mediated degradation and enhancing antiviral signaling; during SenV infection, UFL1 collaborates with 14-3-3ε protein to promote RIG-I pathway activation, accelerating interferon production. However, in the face of Epstein-Barr virus, viral protein BILF1 hijacks the UFMylation system, inducing UFMylation of MAVS protein and targeting it for lysosomal degradation, achieving immune escape. In inflammation regulation, the UFMylation system inhibits LPS or IFN-γ-mediated macrophage activation, reducing the release of pro-inflammatory cytokines such as IL-6 and TNF-α, thereby avoiding excessive immune damage.
- Adaptive Immunity: Key Regulators of Tumor Immunity and Humoral Immunity
In the realm of tumor immunity, the UFMylation system plays a dual regulatory role via the PD-1/PD-L1 pathway: UFL1 promotes UFMylation of PD-L1, accelerating its ubiquitin-mediated degradation and enhancing anti-tumor immunity; however, it simultaneously modifies PD-1, inhibiting its degradation and weakening CD8+ T cell activity. This paradoxical nature provides a theoretical basis for developing combined therapies of "UFMylation modulators + immune checkpoint inhibitors." For example, the combination of UFSP2 inhibitors with PD-1 antibodies can significantly inhibit breast cancer growth. In humoral immunity, the adaptor protein UFBP1 ensures plasma cell development and antibody secretion by inhibiting the PERK pathway, with its deficiency leading to antibody production disorders.
- Cross-System Dialogue: The Secret Link Between Endocrine and Immune Systems
The UFMylation system also acts as a bridge between the endocrine and immune systems: it regulates ER transport of adrenergic receptors, maintaining stress response balance; enhances estrogen receptor stability, influencing reproductive-related immunity; and is highly expressed in the pancreas, protecting pancreatic β cells from ER stress-induced apoptosis, revealing its potential role in metabolic diseases.
III. New Directions in Clinical Translation: From Mechanistic Insights to Drug Targets
This review comprehensively integrates the multidimensional roles of the UFMylation system in immunoregulation for the first time, particularly emphasizing its critical nodal functions in tumor immune escape, viral infection strategies, and autoimmune diseases. Notably, specific interventions in UFMylation enzymes (such as UFL1, UFSP2) are expected to become new breakthroughs in disrupting the tumor immune-suppressive microenvironment and enhancing antiviral immune responses. Zhou Junzhi's team points out that small molecule inhibitors targeting the UFMylation system (such as Compound 8) have entered preclinical research, with future exploration of their synergistic effects with existing therapies paving new avenues for precision medicine.
Researcher Background: Pioneering Exploration in Protein Modification
Corresponding author Dr. Zhou Junzhi, as a high-level talent at Guangdong Medical University, has long focused on novel protein post-translational modifications and the tumor microenvironment. His team has published multiple papers in top international journals, revealing the regulatory mechanisms of UFMylation in tumor metastasis, stem cell aging, and other fields. Currently, they are leading several projects funded by the National Natural Science Foundation of China, dedicated to decoding the clinical application potential of the UFMylation system.
From fundamental molecular mechanisms to complex disease networks, the immunoregulatory code of the UFMylation system is gradually being deciphered. This emerging field not only rewrites our understanding of protein modification and immune interactions but also heralds the advent of an era of precision therapy targeting UFMylation. With the deep integration of basic research and translational medicine, this "new hub in immunology" is poised to become a key to future disease treatment.
Product Information
| Catalog Number | Product Name | Species | Conjugation | Price |
| S0B5348 | Alexa Fluor® 647 Mouse Anti-Human CD279 (PD-1) Antibody (S-R570) | Mouse | Alexa Fluor® 647 | $420 |
| S0B0585 | Rat-Anti Mouse PD-1 Antibody (S-798-5) | Rat | Unconjugated | Inquiry |
| S0B1182 | Mouse Anti-Human CD279 (PD-1) Antibody (S-R570) | Mouse | Unconjugated | $100 |
| S0B5206 | FITC Rat Anti-Mouse PD-1(CD279) Antibody (S-798-5) | Rat | FITC | $85 |
| S0B5182 | Rat Anti-Mouse PD-1 (CD279) Antibody (S-R496) | Rat | Unconjugated | Inquiry |
| S0B1157 | NDUFA1 Recombinant Rabbit mAb (S-1277-177) | Rabbit | Unconjugated | Inquiry |
| S0B0813 | NDUFS8 Recombinant Rabbit mAb (S-1273-10) | Rabbit | Unconjugated | Inquiry |
| S0B0894 | Ndufs4 Recombinant Rabbit mAb (S-1459-14) | Rabbit | Unconjugated | Inquiry |




