The Multifaceted Role of Folate Receptor α in Cancer Therapy Strategies
04 Aug 2025
by AntBio
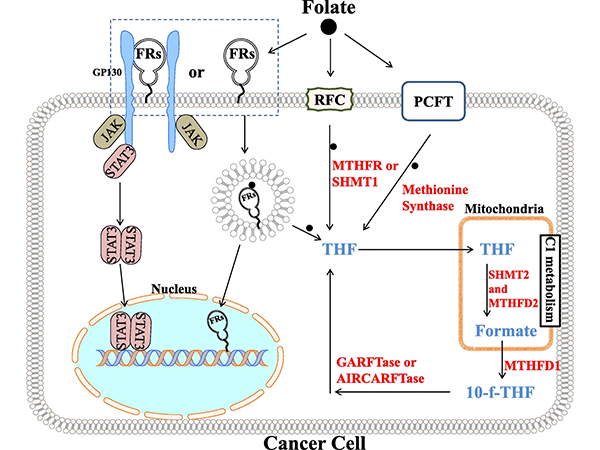
Folate plays a crucial role in DNA synthesis and repair, which are essential for cell proliferation and division. In cancer cells, the aberrant activation of folate one-carbon metabolism may promote tumor growth and survival. Therefore, the folate one-carbon metabolic pathway has emerged as a potential target for cancer therapy.
Among the mechanisms of folate uptake, the most important transporters include the Reduced Folate Carrier (RFC), the Proton-Coupled Folate Transporter (PCFT), and the Folate Receptor α (FRα). These three proteins all play key roles in folate uptake. However, the former two are not currently direct targets of anticancer drugs.
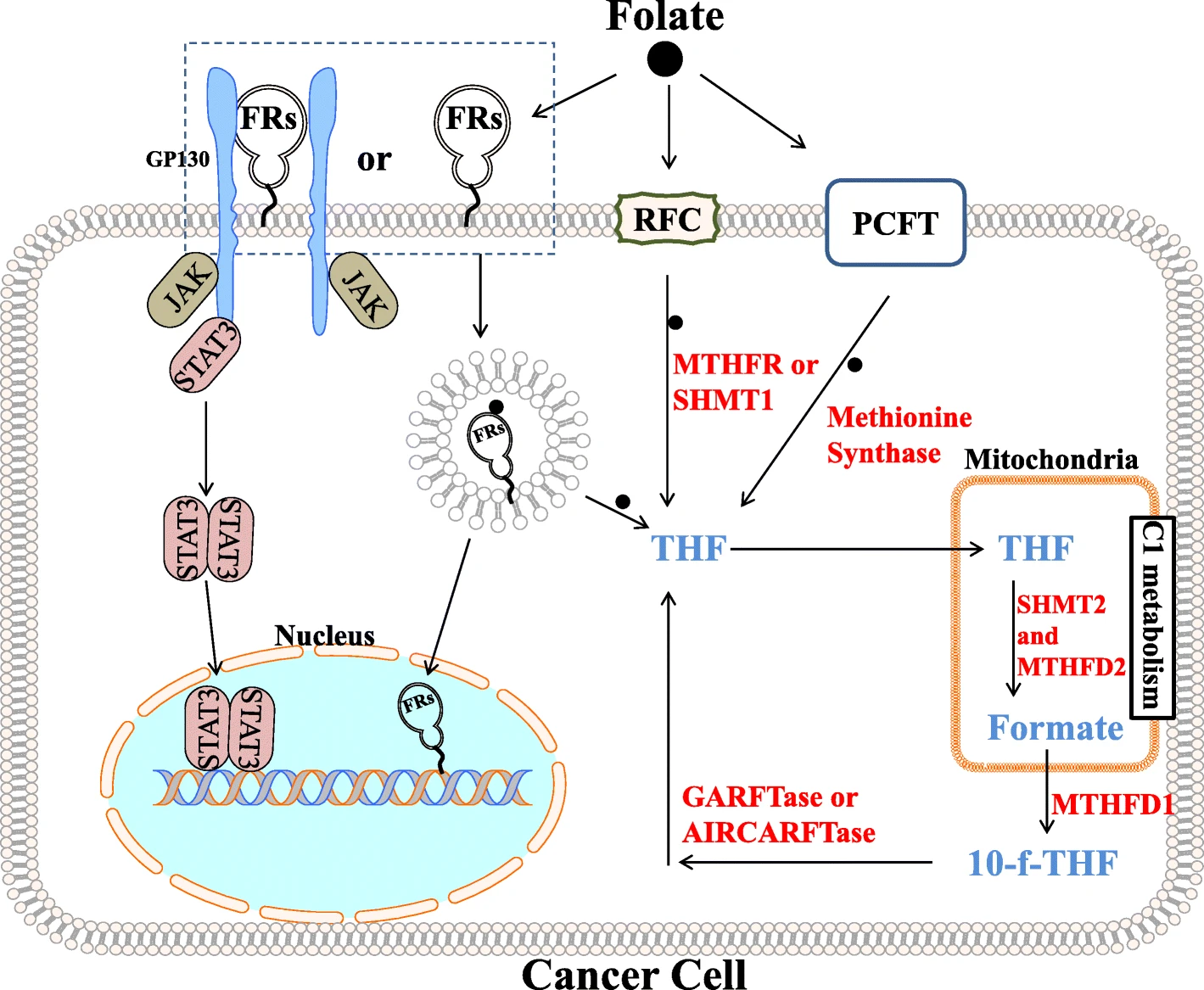
Figure 1: Folate Receptor Signaling and C1 Metabolism
Folate Receptor α (FRα)
Folate Receptor α (FRα), also known as FOLR1 or folate-binding protein, belongs to the high-affinity folate receptor (FR) family and is a 38–40 kDa glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein. It is encoded by the FOLR1 gene, which consists of 7 exons and 6 introns. FRα contains multiple domains, including a folate-binding site and a GPI anchor site, the latter of which is responsible for anchoring FRα to the cell membrane.
FRα has several functional characteristics:
-
Folate Transport: FRα facilitates folate uptake into cells by binding folate and mediating its endocytosis.
-
Tumor Proliferation Regulation: FRα expression levels are significantly increased in certain rapidly proliferating cancer cells, as these cells require more folate to maintain their abnormally high proliferation rates and metabolic activities.
-
Signal Transduction: FRα is involved in tumor invasion, metastasis, and progression and participates in various signaling pathways, such as JAK-dependent activation of STAT3 via the co-receptor GP130 or regulation of PEAK1 phosphorylation through a complex with the LYN tyrosine kinase.
-
Transcription Factor: GPI-anchored FRα can translocate to the nucleus after internalization and function directly as a transcription factor.
Expression of FRα in Various Tissues
FRα is expressed in several non-malignant tissues but is highly overexpressed in certain solid tumors, such as ovarian cancer, lung cancer, colorectal cancer, and breast cancer. Up to 90% of ovarian cancers constitutively express FRα, while it is virtually absent in non-malignant ovarian tissue. It is overexpressed in 76–89% of epithelial ovarian cancers and in 14–74% of non-small cell lung cancers.
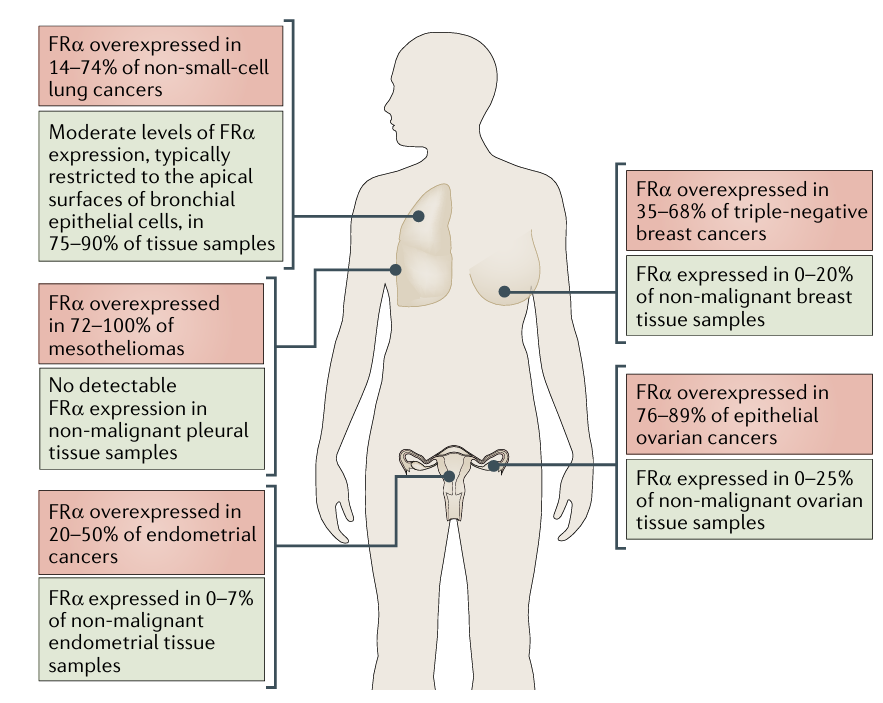
Figure 2: Overexpression of FRα in Cancerous and Non-Malignant Tissues
Development of FRα-Targeted Therapeutics
Given its minimal physiological role in non-malignant tissues post-embryogenesis, overexpression in various cancer types, high affinity for non-physiological substrates (such as folate), and involvement in tumor invasion and metastasis, FRα has emerged as a promising target for cancer imaging and a variety of therapeutic approaches, including antibody drugs, antibody-drug conjugates (ADCs), small molecules, bispecific antibodies, and CAR-T cells.
Imaging and Targeting: FRα is an excellent target for tumor-specific imaging. For instance, Folate-FITC (also known as EC17) is a folate-modified fluorescent probe that enhances the visualization of tumor tissues by exploiting the overexpression of FRα in certain cancer types, such as ovarian cancer, aiding in the identification of metastatic lesions during surgery.
Monoclonal Antibodies: Farletuzumab targets FRα and exerts its effects through several mechanisms:
-
Antibody-Dependent Cellular Cytotoxicity (ADCC): It induces immune cells (such as natural killer cells) to attack and kill FRα-expressing tumor cells.
-
Complement-Dependent Cytotoxicity (CDC): It activates the complement system to destroy tumor cells.
-
Autophagy Induction: Farletuzumab can induce autophagy in tumor cells, a process of cellular self-digestion leading to dysfunction and death. Clinical trials have demonstrated the potential efficacy of Farletuzumab, either as a monotherapy or in combination with other chemotherapeutic agents, for patients with FRα-positive cancers.
Antibody-Drug Conjugates (ADCs): Elahere, an ADC composed of an anti-FRα antibody and a microtubule inhibitor (DM4), is the first ADC approved for ovarian cancer. Preclinical studies have shown its antitumor activity in ovarian and non-small cell lung cancer models, both in vitro and in vivo. Additionally, preclinical in vivo studies have demonstrated antiproliferative effects when Elahere is used in combination with other drugs for ovarian cancer patients, such as bevacizumab, doxorubicin, and carboplatin. On March 22, 2024, the FDA approved ELAHERE for the treatment of specific ovarian cancer patients.
CAR-T Cells: Engineered CAR-T cells incorporating an FRα-specific single-chain variable fragment (scFv) (MOv19) linked to the T-cell receptor chain CD3ζ and a CD137 (4-1BB) co-stimulatory motif have successfully induced tumor regression in preclinical models. These CAR-T cells are currently being tested in Phase I trials. EC17 has also been used to evaluate the activity of CAR-T cells in CAR-T cell therapy, mediated by folate receptor-targeted bispecific aptamer molecules (bispecific adaptor molecules, CAM).

Figure 3: Clinical Applications of FRα-Targeted Therapies in Cancer Diagnosis and Treatment
FRα is a highly relevant and potentially valuable target in cancer therapy, with broad prospects, including new drug development, combination therapy strategies, personalized medicine, and market potential. As research progresses, FRα-targeted therapies hold promise for offering more treatment options and improved outcomes for cancer patients.
S-RMab® Monoclonal Antibodies
Starter has launched a rabbit monoclonal antibody against FRα, which offers higher specificity, sensitivity, and stability, providing more accurate diagnostic results for clinical use.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| S0B2275 | Human folate receptor alpha Recombinant Rabbit mAb (SDT-719-20-2) | Host : Rabbit | Inquiry |
| Conjugation : Unconjugated |




