Introduction to ADC Drug Payloads

In the "arms race" of cancer treatment, antibody-drug conjugates (ADCs) are rewriting the rules of engagement with unprecedented precision and lethality. With over a dozen ADC drugs already approved for market and hundreds of clinical trials in full swing exploring new targets and indications, the revolution is underpinned by four core technological breakthroughs: target validation, antibody optimization, linker upgrades, and payload innovation. Among these, the diversity of payloads serves as the critical engine driving the future evolution of ADCs.
- Payload Evolution: From "Single Warhead" to "Super Weapon Arsenal"
Traditional ADC payloads were akin to "single warheads": microtubule inhibitors (e.g., MMAE) and DNA alkylating agents (e.g., calicheamicin) dominated the landscape. However, the rise of topoisomerase 1 inhibitors in recent years has disrupted this paradigm. The success of Enhertu® (DS-8201a) and Trodelvy® (IMMU-132) demonstrates the potential of non-traditional payloads. These innovations are made possible by breakthroughs in linker technology, enabling higher drug-to-antibody ratios (DAR), more stable payload attachment, and enhanced "bystander effects."
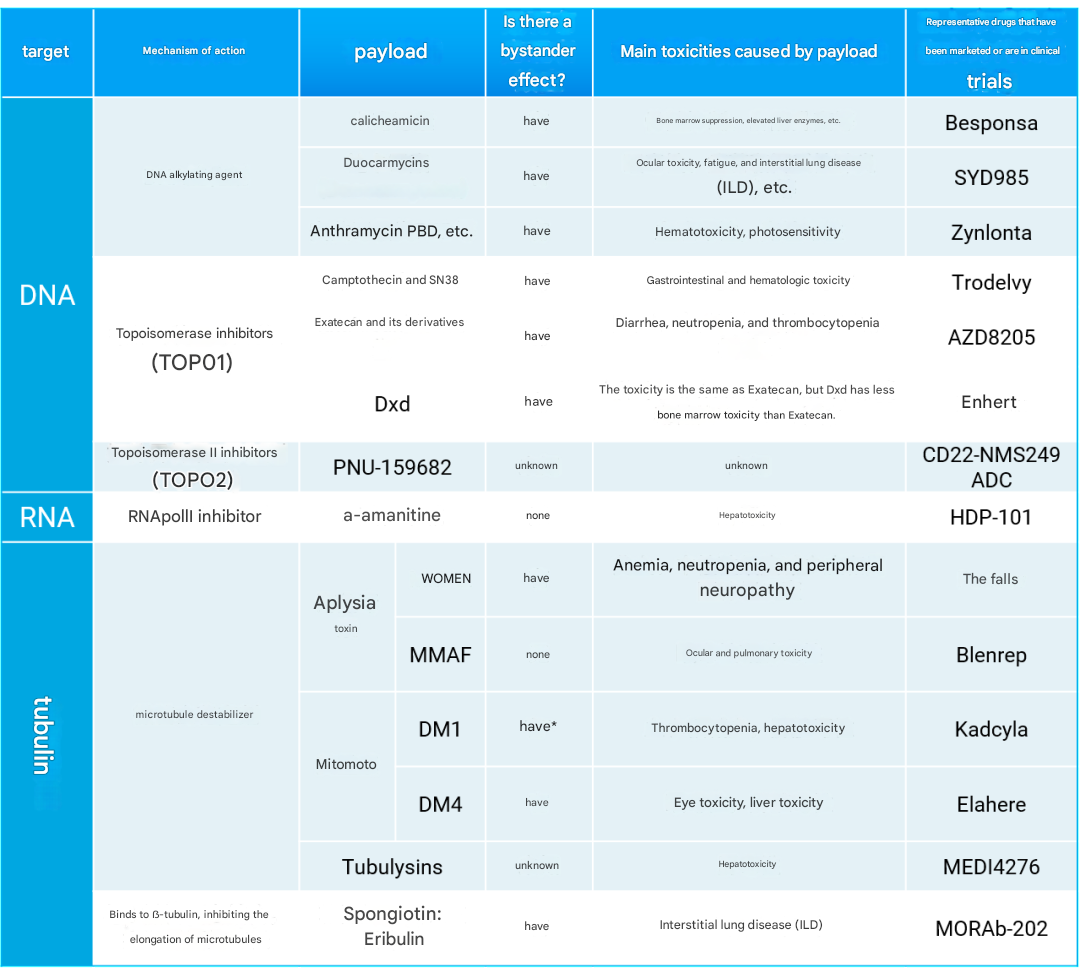
ADC Payload Mechanisms of Action, Bystander Effects, Associated Toxicities, and Drug Examples
- Topoisomerase Inhibitors: "Saboteurs" of DNA Repair Sites
Topoisomerases are the "repair workers" that control DNA supercoiling, and their inhibitors disrupt this repair mechanism, leading to DNA breakage and ultimately inducing cancer cell apoptosis. Although topoisomerase inhibitors are hundreds of times less potent than microtubule inhibitors, ADC structural improvements have addressed this issue:
Camptothecin (CPT) Family
- Enhertu®: Carries DXd (exatecan derivative) with a DAR of up to 8, significantly reducing bone marrow toxicity.
- PRO1184/PRO1160: Employs hydrophilic linkers to overcome hydrophobicity limitations and enhance bystander effects.
- AZD8205: Features the exatecan derivative AZ'0132, targeting B7-H4, demonstrating broad-spectrum potential.
Irinotecan Family
- Trodelvy®: Conjugates SN-38 with TROP2 antibody, approved for triple-negative breast cancer.
- IMMU-130/140: Targets CEACAM5 and HLA-DR respectively, expanding indication boundaries.
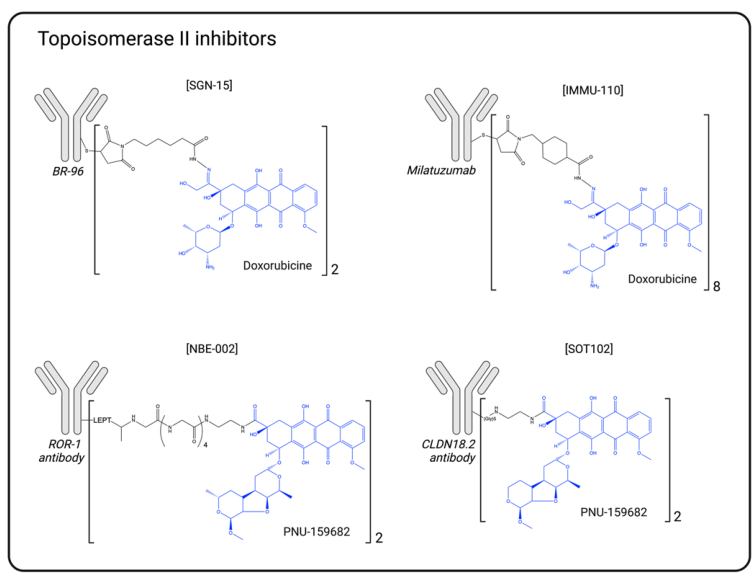
Topoisomerase 2 Inhibitor Schematic
- RNA Polymerase Inhibitors: "Shutdown Buttons" for Transcription Factories
The amanitin family, natural RNA polymerase II inhibitors, can block the transcription process. Despite their intense toxicity, they show unique advantages as ADC payloads:
- HDP-101: A BCMA-targeting ADC that induces immunogenic cell death, synergizing with immune checkpoint inhibitors.
- Other explorations: Triptolide, HDAC inhibitors, and other transcriptional regulatory molecules are breaking through solubility and toxicity bottlenecks.
- Bcl-xL Inhibitors: "Weight Adjusters" of the Apoptosis Balance
Bcl-xL protein acts as a "survival switch" for cancer cells, and its inhibitors trigger cell death by disrupting the pro-apoptotic/anti-apoptotic balance:
- ABBV-155: Conjugates B7-H3 antibody with Bcl-xL inhibitor, demonstrating safety and efficacy in lung and breast cancers.
- Immunostimulatory Payloads: "Alarms" Awakening the Immune System
Inspired by the success of immune checkpoint inhibitors, a new direction has emerged: combining STING/TLR agonists with antibodies for precise release in the tumor microenvironment to activate innate immunity:
- NJH395/BDC1001: Represents a new generation of immunostimulatory ADCs, poised to overcome systemic toxicity limitations.
- Future Battleground: "Limitless Possibilities" in Payload Innovation
The ADC payload library is expanding into broader territories:
- Topoisomerase 2 inhibitors: Such as PNU-159682, overcoming efflux transport limitations.
- Kinase inhibitors: Genistein conjugates showing multi-target potential.
- Dual warhead designs: Crossover combinations like radionuclide + cytotoxin, PROTAC technology, etc.
Conclusion: When "Magic Bullets" Meet "Smart Warheads"
The future of ADC drugs belongs to those who dare to break traditions and embrace innovation. From topoisomerase inhibitors to RNA polymerase inhibitors, from immunostimulatory payloads to dual warhead designs, each new payload category is expanding the boundaries of ADC indications. With technological iterations, ADCs may truly become the "ultimate weapon" to end cancer—precise guidance, intelligent killing, and immune awakening, ushering in a new era of cancer treatment for humanity.




