Guidelines for the preparation of intestinal lamina propria immune cell isolates

1. Preparation before the experiment
Reagent preparation
40% Percoll solution preparation:
Percoll cell isolate solution was mixed with PBS buffer (10×) in a 9:1 volume ratio to prepare an isotonic 100% Percoll mother solution. Prepare a 40% isotonic Percoll solution by mixing 100% Percoll mother liquor and RPMI 1640/1 × PBS in a 4:6 volume ratio.
80% Percoll solution:
Percoll cell isolate solution was mixed with PBS buffer (10×) in a 9:1 volume ratio to prepare an isotonic 100% Percoll mother solution. Prepare an 80% isotonic Percoll solution by mixing 100% Percoll mother liquor and RPMI 1640/1 × PBS in an 8:2 volume ratio.
2. Intestinal lamina propria immune cell isolation steps
1) The mouse was sacrificed by cervical dislocation method and fixed on the operating table, the abdomen of the mouse was disinfected with 75% alcohol, and the abdominal cavity was exposed by longitudinal cutting in the median;
2) As shown in the figure below, cut at the junction of the mouse stomach and duodenum, while separating the adipose tissue and mesentery around the small intestine, pull out the small intestine, and then cut it at the junction of the small intestine and cecum to isolate the small intestine.
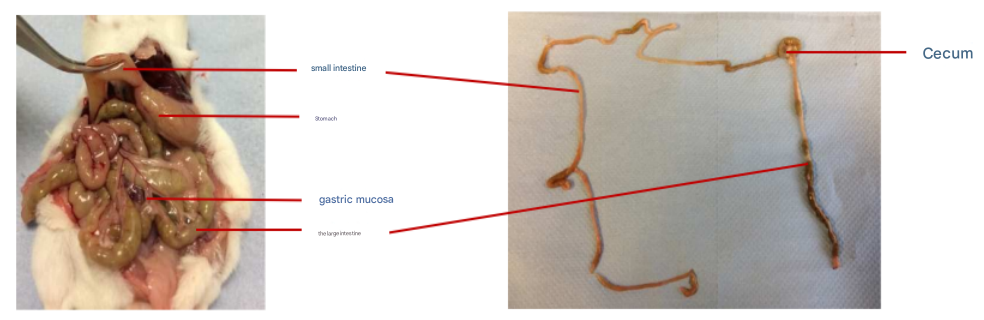
Anatomical diagram of mouse intestinal tissue
Add 5 mL of 4 °C pre-chilled PBS to a 10 cm Petri dish, rinse the small intestine in step 2 into the Petri dish, and use forceps and scissors to remove the adipose tissue and Peyle node sequentially.
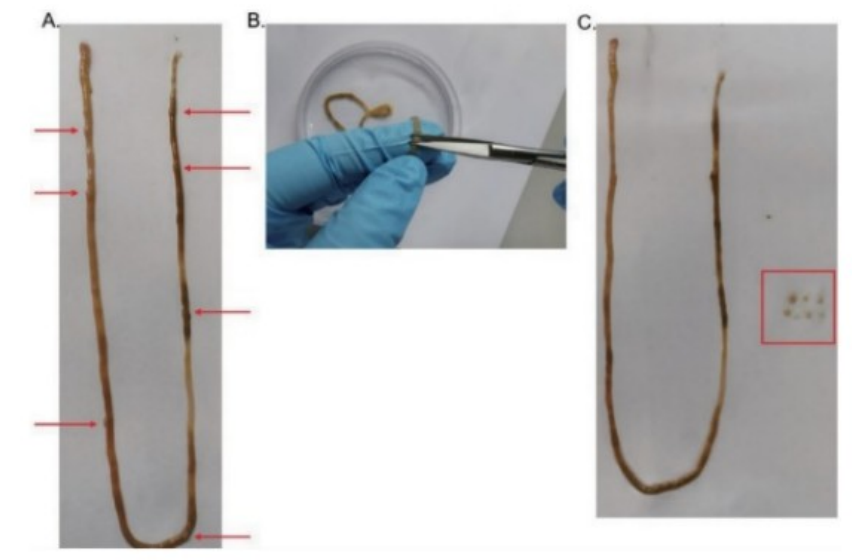
Mouse Payle junction removal operation diagram
A. Small intestine with removal of tissues such as surface fat and mesentery, with red arrows indicating Patrick's knots (an average of 6-9 Patrick's knots per mouse);
B. Schematic diagram of the operation of cutting off the Patrick knot;
C. Small intestine after removal of the Patrick's knot, the red box is all the Piper knots that have been cut out.
Peyer's patches (PPs) are an important part of the mouse intestinal mucosal immune system, mainly distributed in the lower part of the mouse small intestine, i.e., the distal jejunum and ileum, and are significantly different from the composition and function of mouse lamina propria immune cells.
4) cut the small intestine into 4 sections, cut the small intestine longitudinally with scissors (not necessarily in the direction of the mesentery), expose the intestinal contents, and gently scrape the intestinal contents from the surface of the mucosa;
5) Transfer the small intestine to a 50 mL centrifuge tube containing 10 mL of HBSS + 2% FBS and vortex vigorously for 10-20 s;
6) Take out the small intestine with tweezers and repeat the cleaning 2 times;
7) The cleaned intestinal tissue was cut into 0.5cm small fragments, the tissue block (100-200mg) was transferred to a 2mL round-bottom tube, 1mL of mouse intestinal tissue dissociation solution (abs50093) was added, and the incubation was incubated with gentle shaking on a horizontal shaker at 37°C for 60min (the incubation time was appropriately extended according to the state of tissue erosion);
8) Remove the round-bottom tube from the horizontal shaker, vortex vigorously for 10-20s, filter through a 70 μm cell sieve (abs7232), and rinse the cell sieve with 10 mL of PBS;
9) Collect the filtrate containing cells into a 50 mL tube, centrifuge at 400 g at 20 °C for 5 min, discard the supernatant, resuspend the cell pellet with 4 mL of 40% percoll (abs9102) and transfer to a 15 mL centrifuge tube;
10) Pipette 2.5 mL of 80% percoll deep into the bottom of the 15 mL centrifuge tube, tilt the tube slightly, and slowly add 4 mL of the mixture of 40% percoll with cells along the wall of the tube to the centrifuge tube, covering the top of the 80% percoll solution to form a density gradient;
▲ Note: Do not disrupt the interface between 80% percoll and 40% percoll and the suspension of cells, 40% and 80% percoll are ready to use.
11) Centrifuge at 800 g at 20 °C for 20 min, speed up (ACC) 1, speed down (DEC) 1;

Schematic diagram of Percoll isolation of tissue immune cells
Note: The separation image is referenced from DOI: 10.21769/BioProtoc.1010327
12) After centrifugation, pipette the immune cells in the middle buffy layer into a new 15 mL centrifuge tube, add 3 times the volume of pre-cooled PBS at 4°C, mix it upside down, centrifuge at 400 g at 4 °C for 5 min, and discard the supernatant;
13) Repeat the wash once according to step 12, discard the supernatant to obtain intestinal lamina lamina propria;
14) Resuspend the cell pellet in cell staining buffer (ABS9475) and adjust the cell concentration to 5-10×106 cells/mL, dispense 100 μL/tube of the cell suspension into a 12×75 mm flow cytometry tube (abs7303) for subsequent experimental manipulation.
3. Flow cytometry experimental results
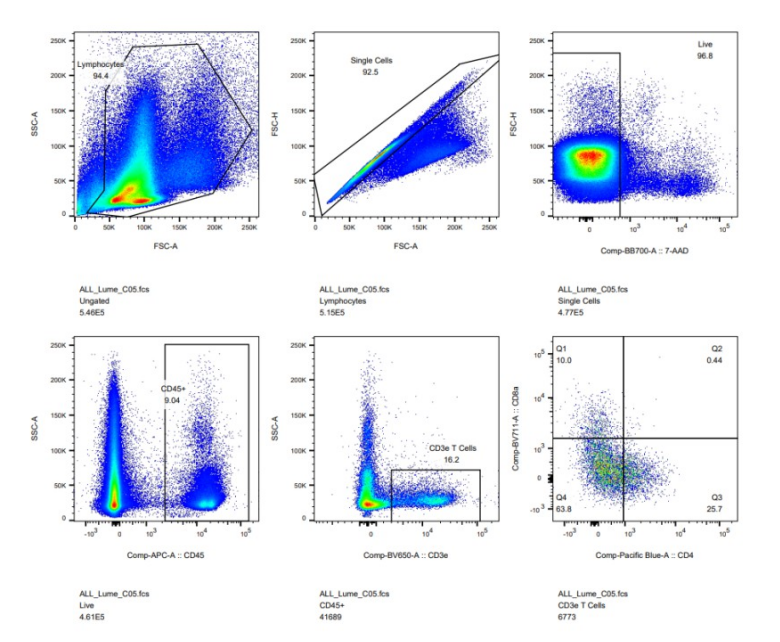
4. Related product recommendation
|
Catalog number |
name of article |
specification |
use |
|
abs9474 |
Tissue preservation solution |
100mL |
Save the organization |
|
abs50093 |
Mouse intestinal tissue dissociation kit |
25T |
Digestive tissue enzymes |
|
abs9102 |
Cell separation fluid |
100mL |
Enrichment of cells |
|
abs7232 |
70um cell sieve |
100 pcs |
Screens |
|
abs7303 |
5mL flow cytometry tubes (sterile, enzyme-free, capped) |
500 pcs |
Flow cytometry tubes |
|
abs9475 |
Cell staining buffer |
500mL |
Staining buffer |
More streaming good things are recommended
|
Catalog number |
Product name |
specification |
|
abs9907 |
Flow cytometry absolute counting tubes |
50T |
|
abs9477 |
Mouse Fc receptor blocker |
200T/500T |
|
abs9476 |
Human Fc receptor blockers |
50T/200T |
|
abs9488 |
Sheath fluid |
20L |
|
abs9107 |
Phoboester |
1mg/5mg/25mg |
|
abs9108 |
Ionomycin calcium salt |
1mg/5mg |
|
abs970 |
D-PBS buffer (1 ×, no calcium magnesium) |
500mL |
|
abs810012 |
Brefeldin A |
10mg/25mg/50mg |
|
abs812975 |
Monensin sodium salt |
25mg/100mg |
|
abs9110 |
fixer |
50mL |
|
abs9111 |
Permeabilization agent |
150mL |
|
abs7303 |
5 mL flow cytometry tubes (sterile, enzyme-free, with caps) |
1 box |
|
abs1840002 |
APC Mouse anti-Human CD3 Antibody(3JI3-9) |
25T/100T |
|
abs182427 |
PE Mouse anti-Human CD4 Antibody(5D4) |
25T/100T |
|
abs182430 |
FITC Mouse anti-Human CD8 Antibody(3C4) |
25T/100T |
|
abs180046 |
PE-Cy7 Mouse anti-Human CD25 Antibody(BC96) |
25T/100T |
|
abs1840696 |
FITC Mouse anti-Human CD80 Antibody(2D10.4) |
25T/100T |
|
abs182338 |
APC Rat anti-Mouse CD25 Antibody(4APC61.5) |
25T/100T |
|
More...... |
||
Absin provides antibodies, proteins, ELISA kits, cell culture, detection kits, and other research reagents. If you have any product needs, please contact us.
|
Absin Bioscience Inc. |
 Follow us on Facebook: Absin Bio Follow us on Facebook: Absin Bio |




