Decoding IBD: The Immunoregulatory Mechanism of TL1A/TNFSF15 and DR3 Signaling Pathways

Abstract
TNF-like cytokine 1A (TL1A), also known as TNFSF15, is a member of the tumor necrosis factor family. It is expressed in various immune cells (such as monocytes, macrophages, dendritic cells, and T cells) and non-immune cells (such as synovial fibroblasts and endothelial cells). By binding to death receptor 3 (DR3), TL1A regulates downstream signaling pathways, influencing the proliferation, activation, apoptosis of effector cells, as well as the production of cytokines and chemokines.
Recent studies have found that TL1A is abnormally expressed in various autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease (IBD), psoriasis, primary biliary cirrhosis, systemic lupus erythematosus, and ankylosing spondylitis, and plays a role in the pathogenesis and progression of these diseases.
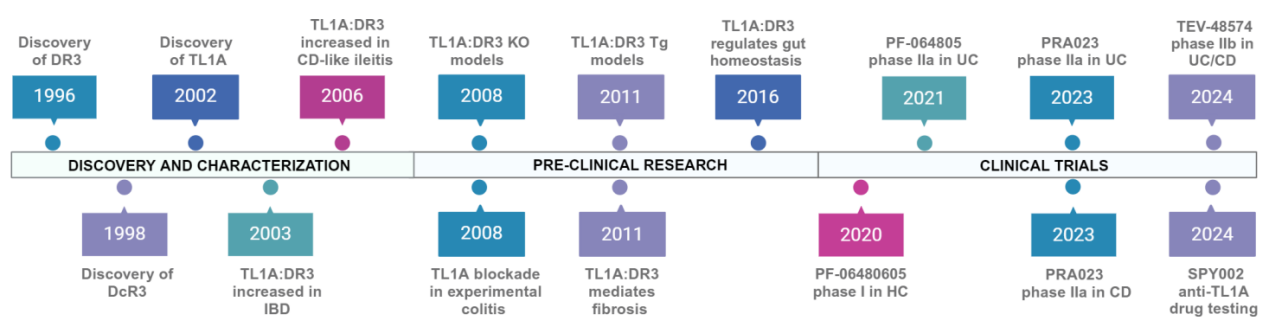
Timeline of Anti-TL1A/DR3 Therapeutic Development
Biological Functions
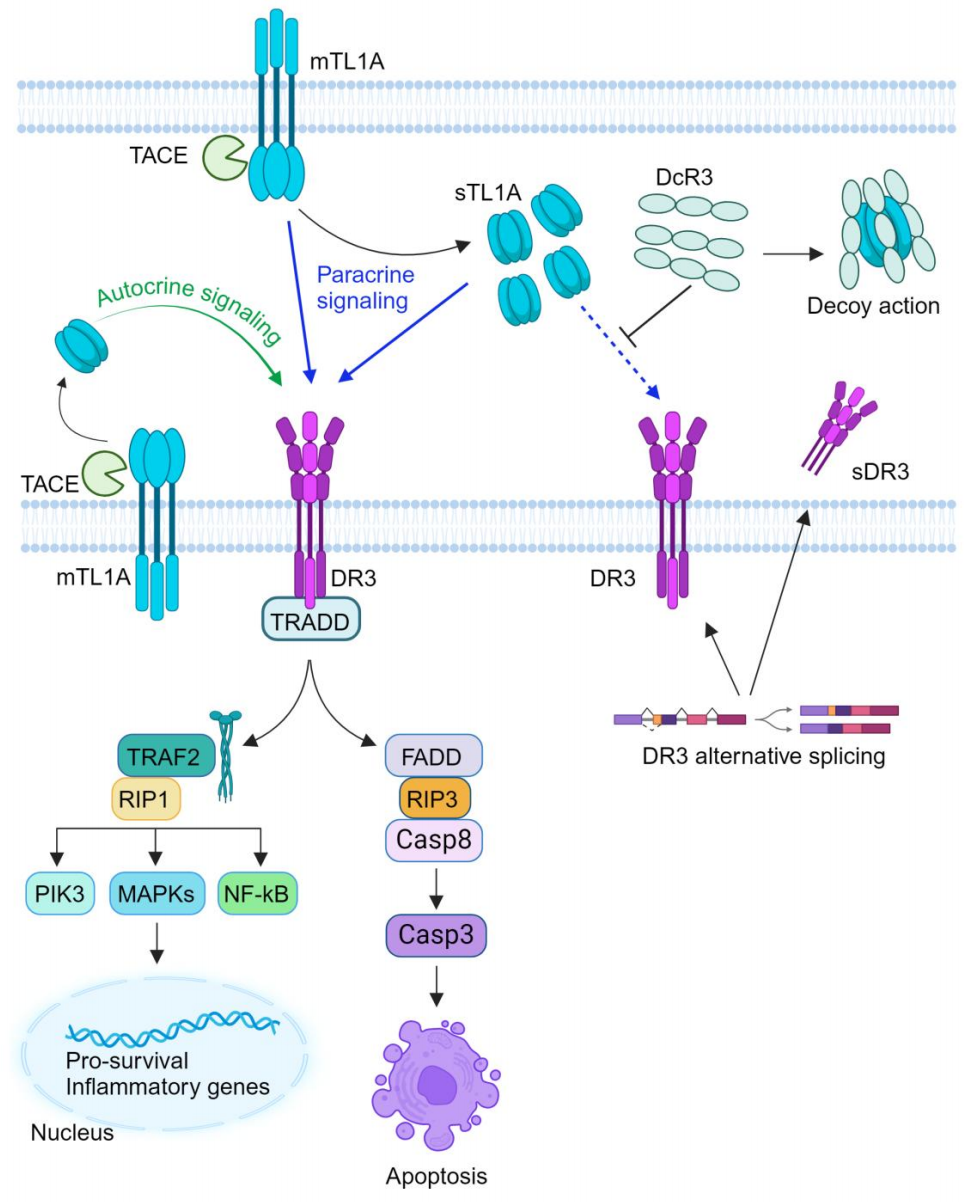
Through its binding with DR3, TL1A activates downstream signaling pathways, including mitogen-activated protein kinases (MAPKs), nuclear factor kappa B (NF-κB), and the effector kinase PI3K signaling pathway, thereby regulating the expression of pro-inflammatory genes and the occurrence of immune-related diseases. The interaction between TL1A and DR3 may trigger two distinct signaling pathways, leading to inflammation and apoptosis, respectively. Additionally, TL1A can bind to TNFR2, producing pro-inflammatory components such as IL-6 and reactive oxygen species (ROS), which further affect the mitochondrial function of fibroblast-like synoviocytes (FLSs).
Role in Diseases
TL1A/TNFSF15 is associated with various inflammatory diseases, particularly IBD. Genome-wide association studies have identified polymorphisms in the TL1A gene linked to an increased susceptibility to IBD. Furthermore, research has shown elevated TL1A RNA levels in tissue biopsies of IBD patients and increased DR3 expression on CD4+ T cells. The abnormal expression of TL1A/TNFSF15 in IBD, along with evidence that blocking the TL1A/DR3 pathway can mitigate inflammatory responses in multiple autoimmune diseases—including IBD, rheumatoid arthritis, and psoriasis—highlights its significance.
Expression of TL1A in Inflammatory Autoimmune Diseases
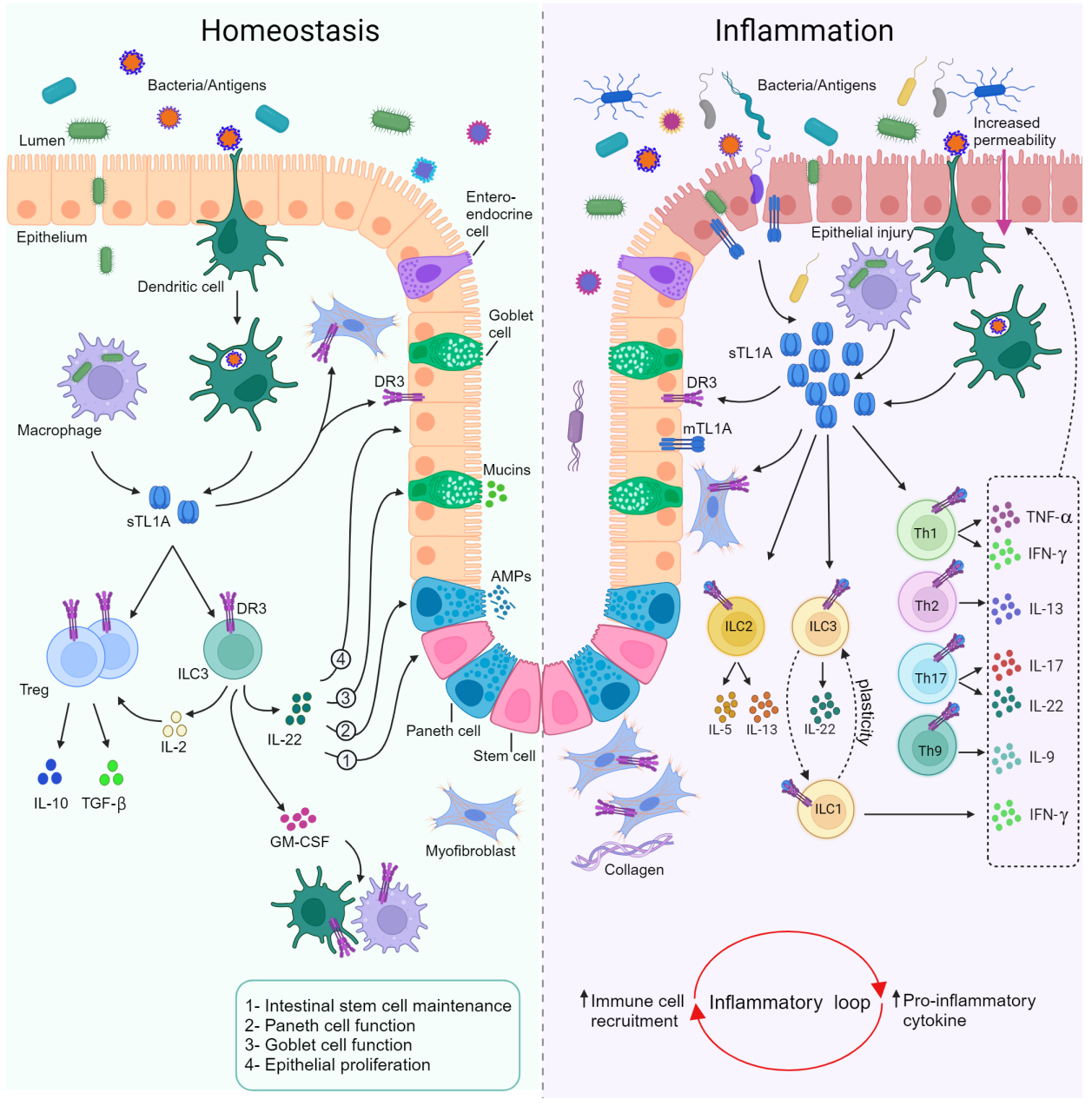
TL1A:DR3 is a key regulator of homeostasis and inflammatory mucosal pathways. The interaction between TL1A and DR3 contributes to several homeostatic processes in the intestinal mucosa. Intact TL1A:DR3 signaling appears essential for the smooth recovery of acute intestinal wall injury through various mechanisms. Under such triggers, TL1A-expressing cells, such as dendritic cells (DCs) and CX3CR1+ mononuclear phagocytes, interact with DR3-expressing homeostatic cell populations to restore a healthy mucosal state. These TL1A-mediated responses include enhanced proliferation of regulatory T cells (Tregs), increased secretion of regulatory cytokines, maintained suppressive function, secretion of the epithelial barrier-protective cytokine IL-22 by gut-resident RORγt+ group 3 innate lymphoid cells (ILCs), and direct effects on the epithelial compartment.
This process is highly influenced by microbial-derived signals, which have been shown to modulate TL1A expression on monocytes and DCs. Additionally, TL1A:DR3 engagement in monocytes is necessary for optimal bacterial uptake and intracellular bacterial clearance, indicating the presence of locally regulated homeostatic responses. The onset of intestinal inflammation is associated with a marked upregulation of TL1A and DR3 in the mucosa, which acts as a potent amplifier of inflammatory pathways. Activated lymphocytes express DR3 and respond to APC-derived TL1A, culminating in the generalized co-stimulation of effector immune pathways. Effector T cells (Teff) and their corresponding cytokines serve as key pathogenic modules that perpetuate mucosal inflammation in IBD.
Pathogenic ILCs are similarly affected and contribute to a pro-inflammatory mucosal environment rich in IL-13/IL-5 secreted by ILC2s and IFNγ secreted by ILC1s. Inflammatory chemokines are also elevated and may promote the recruitment of inflammatory cells to the intestinal mucosa, creating a self-amplifying cycle of cell recruitment and inflammatory mediator production. Overall, current evidence supports the concept that TL1A:DR3 acts within a complex cellular and molecular network, with the net effect determining the balance between homeostasis and inflammation in the intestinal mucosa.
Future Research Perspectives
Currently, TL1A is considered the sole ligand that binds to DR3 and induces functional signaling, playing a pivotal role in a wide array of cellular and molecular pathways. However, several lines of evidence challenge the exclusivity of this binding. DR3 and TL1A deficiencies do not produce identical spontaneous or induced immune phenotypes, and their impacts on the dynamics and outcomes of experimental inflammation also differ. Moreover, the existence of other DR3 ligands has been demonstrated under certain conditions.
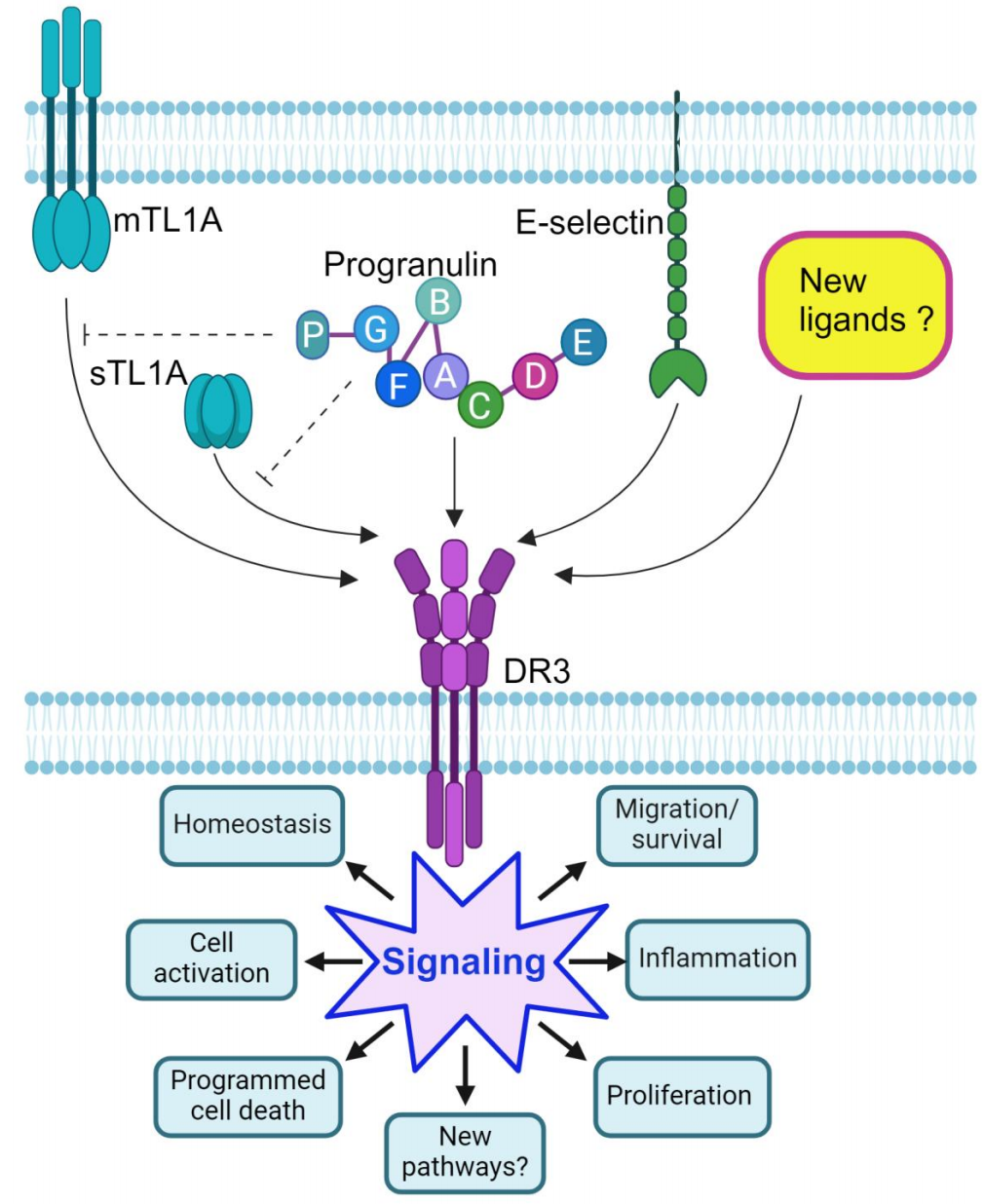
DR3 is expressed on HT29 intestinal epithelial cells (IECs) and is associated with E-selectin and Atsttrin, which, in addition to TNFR1 and TNFR2, can also bind to DR3. The validation and discovery of other DR3 ligands will undoubtedly explore the intriguing possibility that targeting DR3 may offer an alternative—and potentially more effective—anti-inflammatory strategy compared to anti-TL1A approaches. This would justify the development and testing of DR3-targeted therapies for human immune-mediated diseases.
Through in-depth research on the TL1A protein, we can gain a better understanding of its role in cellular processes and diseases, providing robust support for the development of new therapeutic methods and drugs. Simultaneously, research on TL1A will bring new breakthroughs and advancements to the field of biomedicine.
Product Information
| Gatalog Num | Product Name | Product Parameters | Price |
| UA011084 | DR3/TNFRSF25 His&Fc Tag Protein, Human | Host : Human | $600 |
| Expression System : HEK293 | |||
| Conjugation : Unconjugated | |||
| UA040184 | TL1A/TNFSF15 Protein,Human | Host : Human | $112 |
| Expression System : E.coli | |||
| Conjugation : Unconjugated |