Product Details
Product Details
Product Specification
| Host | Rabbit |
| Synonyms | Neurofibrillary tangle protein, Paired helical filament-tau (PHF-tau) |
| Immunogen | Synthetic Peptide |
| Accession | P10636 |
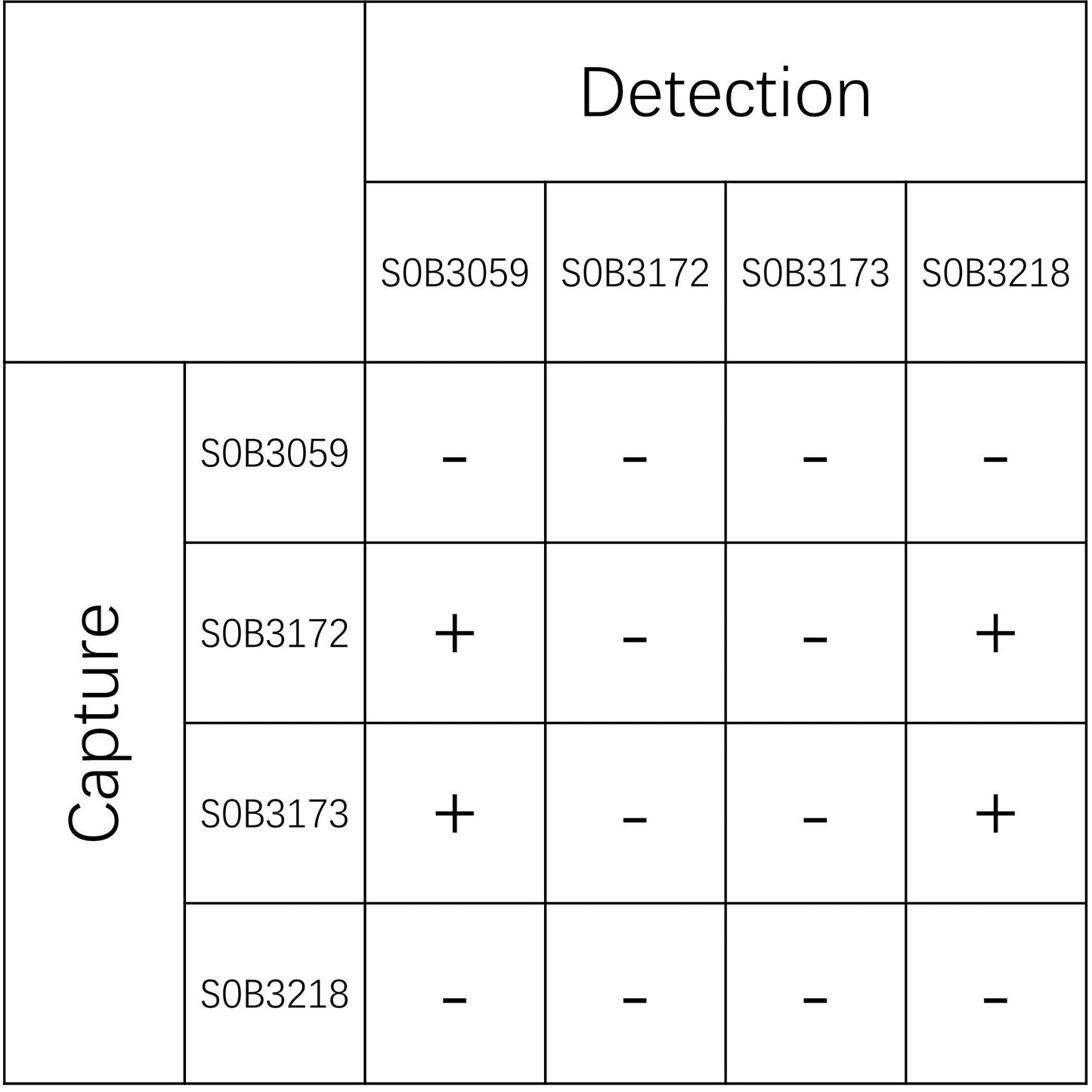
| Clone Number | SDT-173-26 |
| Antibody Type | Recombinant mAb |
| Isotype | IgG |
| Application | Sandwich ELISA |
| Reactivity | Hu, Ms |
| Predicted Reactivity | Ms |
| Cross Reactivity | Recognize all six Tau isoforms |
| Purification | Protein A |
| Concentration | 2 mg/ml |
| Purity | >95% by HPLC |
| Conjugation | Unconjugated |
| Physical Appearance | Liquid |
| Storage Buffer | PBS pH7.4, 0.03% Proclin 300 |
| Stability & Storage | 12 months from date of receipt / reconstitution, 2 to 8 °C as supplied |
Dilution
| application | dilution | species |
| Sandwich ELISA | N/A |
Background
Tau, a microtubule-associated protein, plays an important role in the normal function of neuron. Its phosphorylation promotes axonal and synaptic plasticity in the developing brain. However, under pathological condition, hyper-phosphorylation and aberrant assembly of tau protein result in insoluble aggregates which are accompanied by synaptic dysfunction and neural cell death. Neurodegenerative conditions like Alzheimer's disease (AD) and some forms of frontotemporal dementia are examples of tau-pathies. Tau protein is considered as a promising candidate biomarker for axonal degeneration and neurofibrillary tangle (NFT) formation in AD. However, there are several challenges for molecular characterization of tau in cerebrospinal fluid (CSF). First, in the adult human brain, there exist six different tau isoforms produced from a single gene. Second, this heterogeneity is compounded by extensive posttranslational modifications, including phosphorylation, glycosylation, and oxidation of the protein. There are several serine and threonine phosphorylation sites in tau protein, and phosphorylation is observed in different locations in different diseases. Additionally, concentration of tau in CSF is only 300 ng/ML in healthy individuals and 900 ng/ML in AD subjects. Considering that this quantity is distributed over many different modified forms and six splice variants, the amount available for analysis of each molecular species remains a challenge. However, recent methodologies have enabled for detection of total and phosphorylated tau (P-tau) in CSF. Several longitudinal studies suggest tau pathology as downstream of the amyloidogenic cascade in AD. Longitudinal studies of carriers of mutation of autosomal dominant genes of AD long before the appearance of symptoms provided insight about how Alzheimer's pathology develops in the brain. This has helped in the postulation of AT (N) hypothesis that suggests that amyloid and tau played an orchestrated role in AD pathogenesis. The search for the early biomarkers has come a long way thereafter. The chemical analysis of CSF to demonstrate low Aβ-42 peptide and high P-tau and total tau (T-tau) and their ratios has offered easy differentiation from other diseases and making a diagnosis of AD. CSF Aβ-42 and P-tau levels are considered as surrogate markers for the amyloid and tau deposition in the brain, which have been proven with correlation studies with amyloid and tau imaging. The attempt to provide a diagnosis in early symptomatic stage as in mild cognitive impairment (MCI) is essential to provide disease-modifying therapies. The anti-amyloid and anti-tau therapies are the key agents that are considered to prevent ongoing neurodegeneration and halt the progression. Although these therapies are yet to be available for regular use in clinical practice, advancement in diagnostics is happening very fast to welcome these therapies.
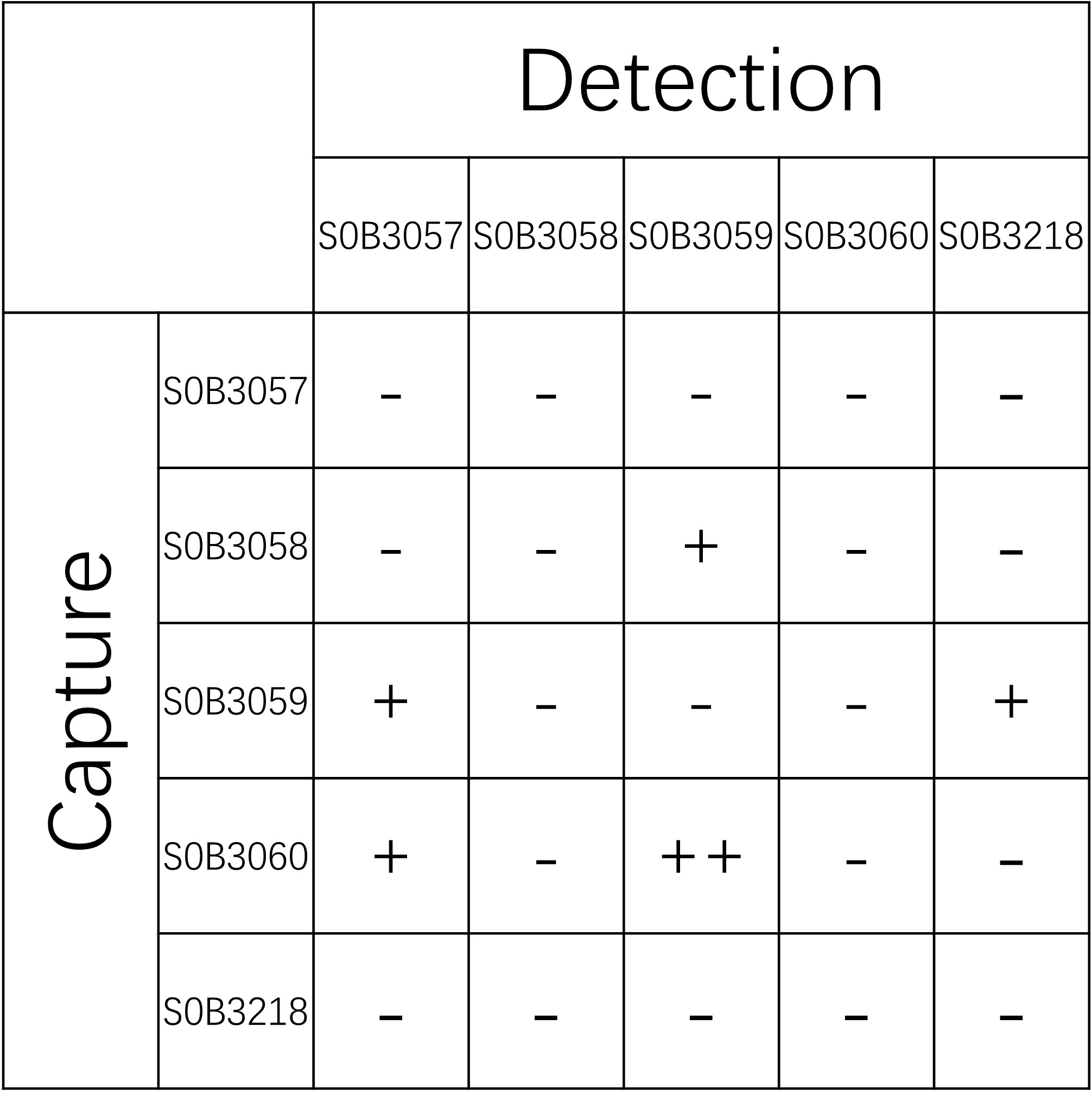
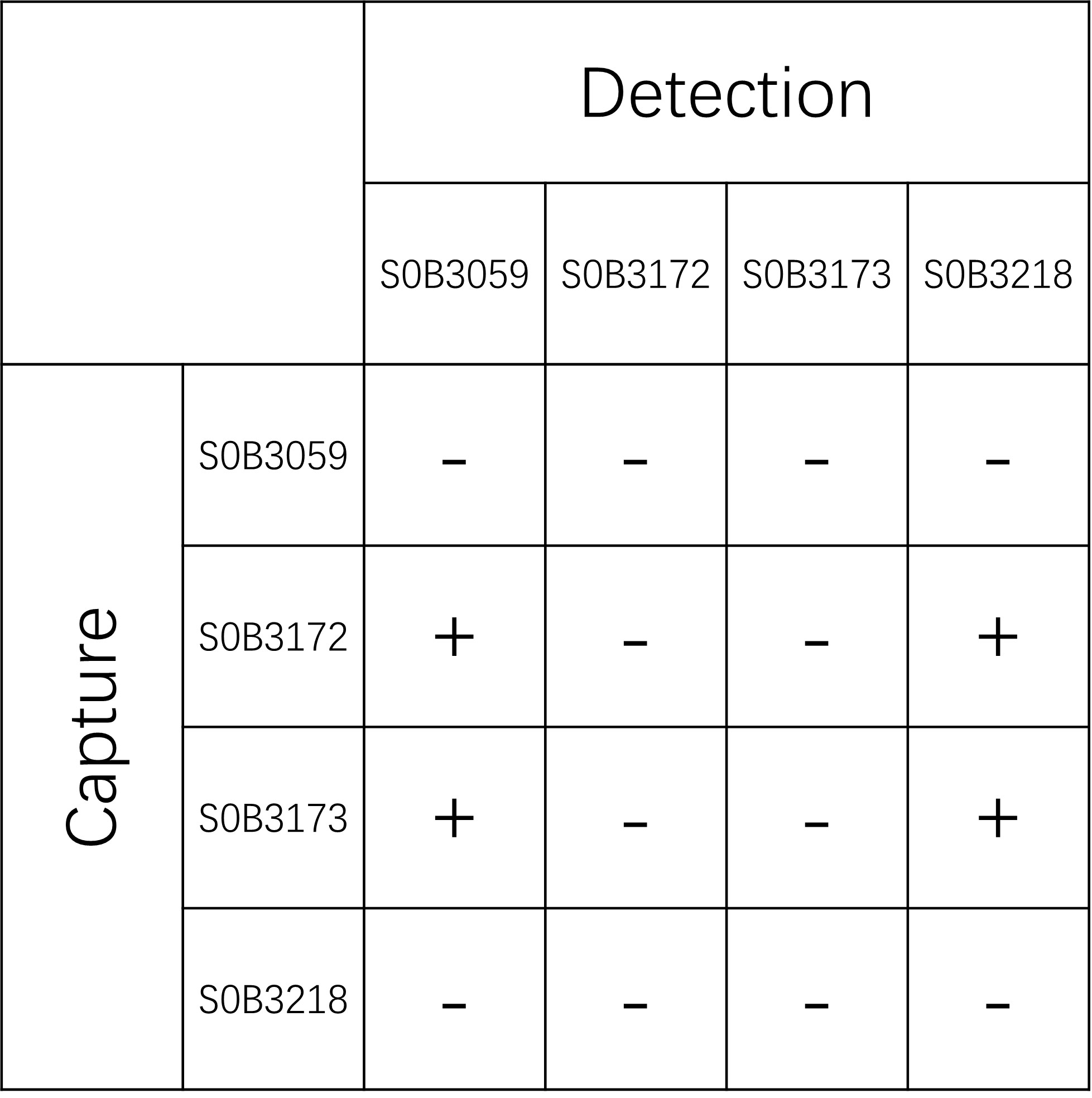
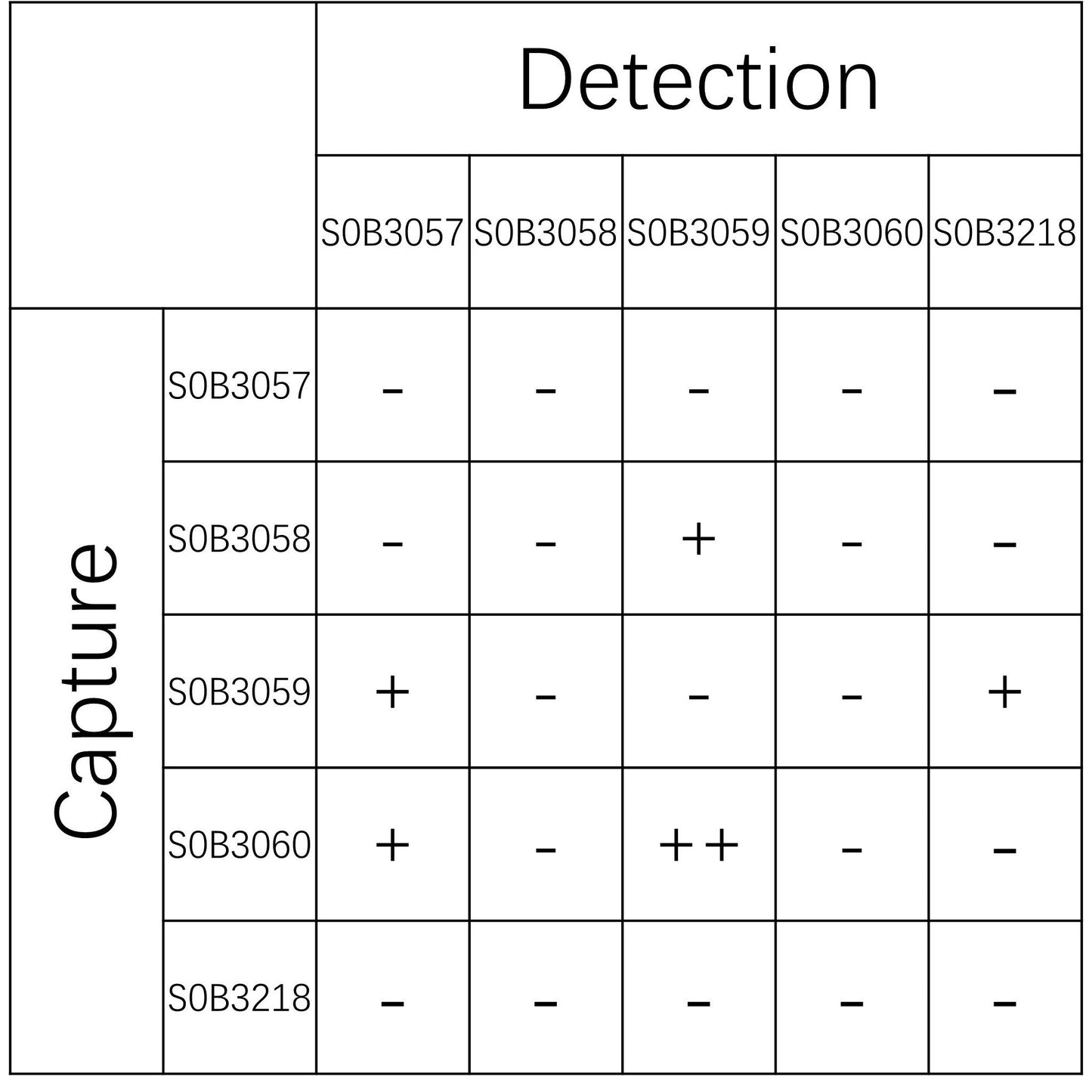
Picture
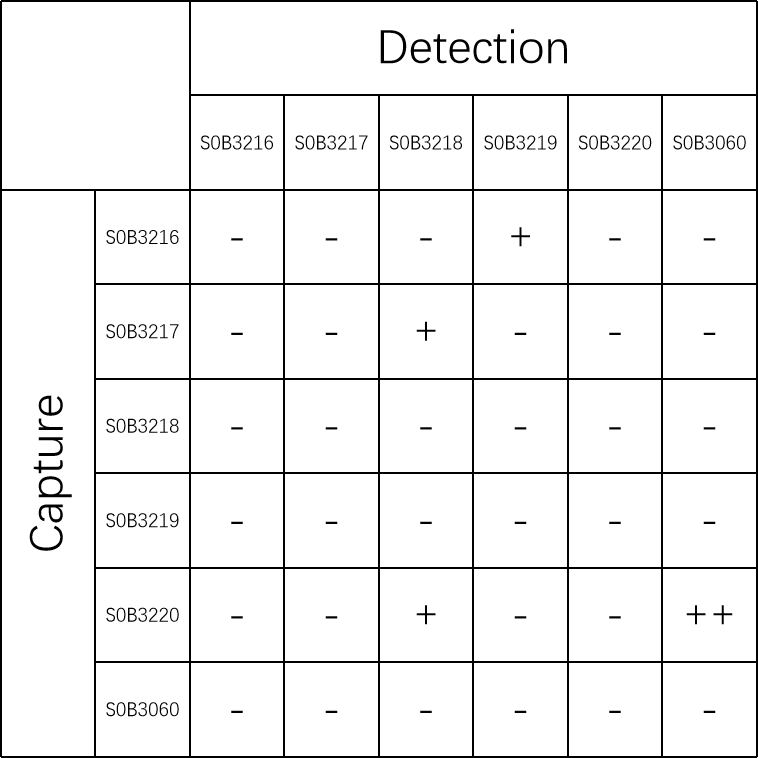
Picture
Paired Recommendations