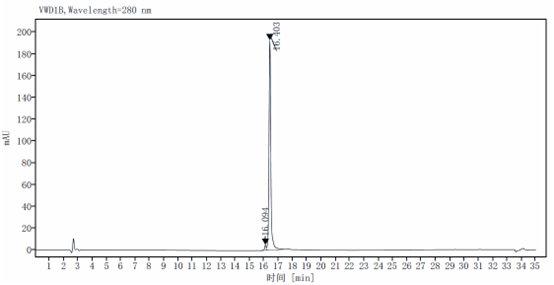
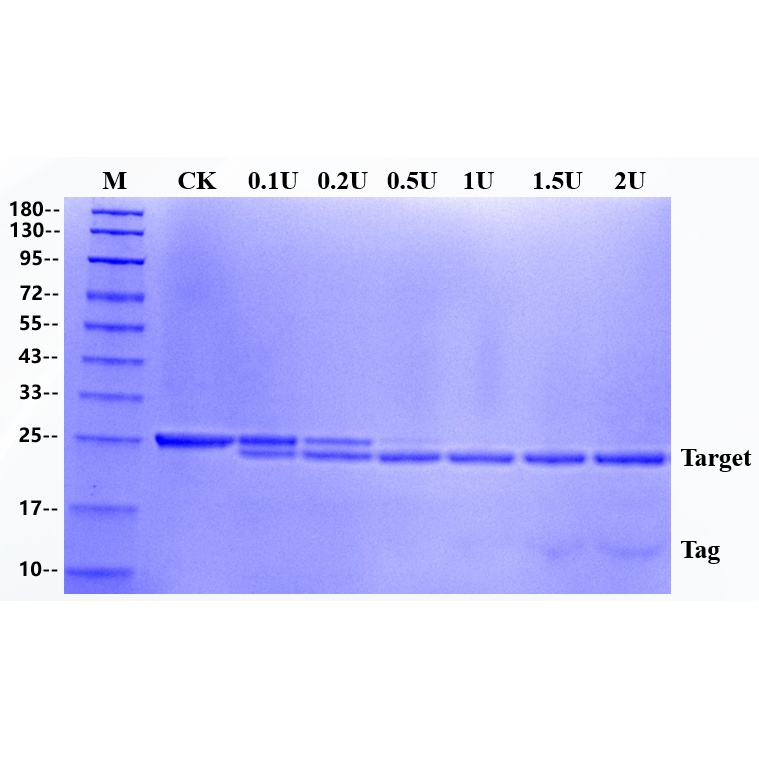
The substrate of 3 μg fusion protein was digested by enzyme, and the addition amount of rTEV Protease in the sample was 0.1U, 0.2U, 0.5U, 1U,1.5U and 2U, respectively. The substrate is a fusion protein with a molecular weight of 25 KD and reacted at 30 ℃ for 1 hour, and the target band of 23kD is produced after rTEV Protease digestion.
Product Details
Product Details
Product Specification
| Species | Tobacco Etch Virus |
| Synonyms | rTEV,TEV,RTEV |
| Concentration | 1U/μ、10U/μl |
| Expression System | E.coli |
| Molecular Weight | 28 kDa (Reducing) |
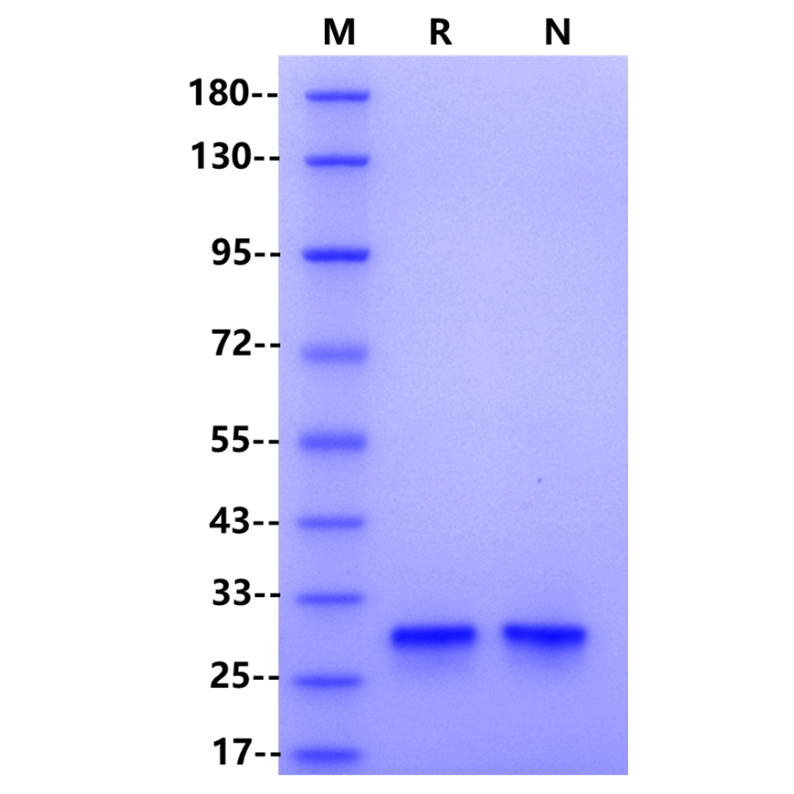
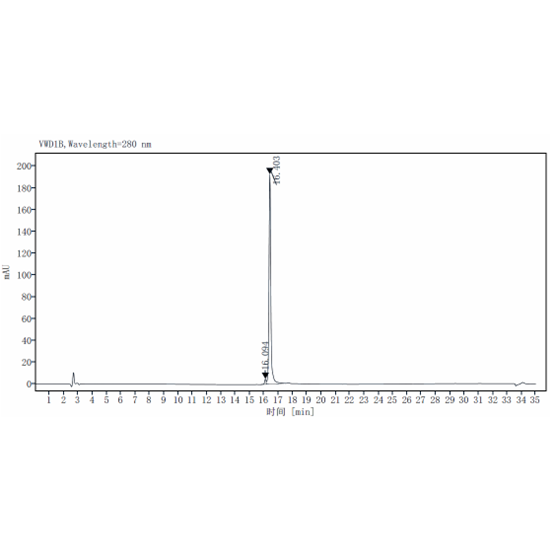
| Purity | >98% by SDS-PAGE & RP-HPLC |
| Endotoxin | <1EU/μg |
| Conjugation | Unconjugated |
| Tag | His Tag |
| Physical Appearance | Liquid |
| Storage Buffer | 20mM Tris-HCl, pH8.0, 150mM NaCl, 1mM EDTA, 5mM DTT, 50%(v/v) Glycerol |
| Stability & Storage | · 12 months from date of receipt, -20 to -70 °C as supplied. |
Background
The tobacco etch virus (TEV) protease is a useful tool for the removal of fusion tags from recombinant proteins. TEV protease has a strict 7 amino acid cleavage recognition sequence of Glu-Asn-Leu-Tyr-Phe-Gln-Gly/Ser [ENLYFQ(G/S)] and cleavage occurs between the Gln and Gly/Ser residues, The most commonly used sequence is ENLYFQG. It is recommended that the cleavage for each fusion protein be optimized by varying the amount of recombinant viral TEV protease, reaction time, or incubation temperature. It can be removed by Ni2+ affinity resin. Recombinant Tobacco Etch Virus Protease (rTEV) has (NIa) protease catalytic domain which corresponds to a molecular weight of 28 kDa. It is unique with high specificity and is active at low temperature. rTEV Protease has a 6*His-tag for easy removal from a reaction using nickel affinity resins and has been engineered to improve thermal stability and decrease autolysis.
Components
- TEV Protease;
- 10X TEV Protease Buffer + Salt: 500mM Tris-HCl, pH8.0, 500mM NaCl, 5mM EDTA, 10mM DTT;
- 10X TEV Protease Buffer – Salt: 500mM Tris-HCl, pH8.0, 5mM EDTA, 10mM DTT;
Protocol
1. Add the following to a microcentrifuge tube:
Fusion Protein |
15μg |
10X rTEV Protease Buffer +/– Salt
|
5μl |
rTEV Protease
|
5U
|
ddH2O
|
To 50μl |
2. Mix and incubate at 30°C, Remove 10 μL aliquots at 1, 2, 4, and 6 hours.
3. Analyze by SDS-PAGE.
Guidelines for Cleavage
- For most fusion proteins, TEV Protease functions optimally in a reaction mixture without NaCl; however, conditions may be optimized by varying the NaCl concentration from 0 mM to 500 mM. Remember to take into account the contribution of salt from the enzyme and from your substrate. When setting up your cleavage reaction, use the appropriate 10X rTEV Protease Buffer +/- Salt.
- Researchers need to optimize their specific reaction conditions. As an initial suggestion, 10 units of rTEV protease can be used per 30μg of target protein for 1 hour at 30 °C, or overnight at 2–8 °C. The cleavage efficiency can then be estimated by SDS-PAGE.
Unit Definition
Picture
Picture
Bioactivity
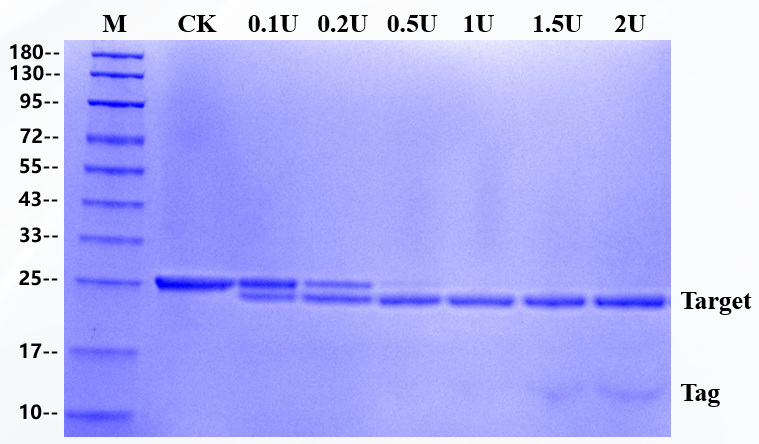
SDS-PAGE
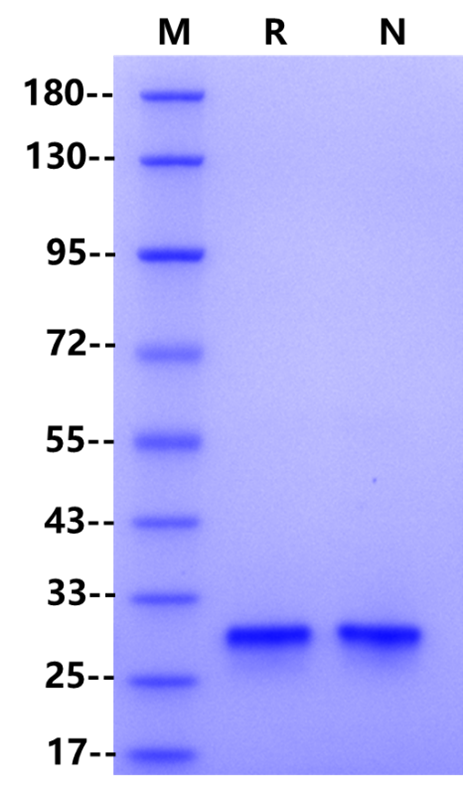
RP-HPLC