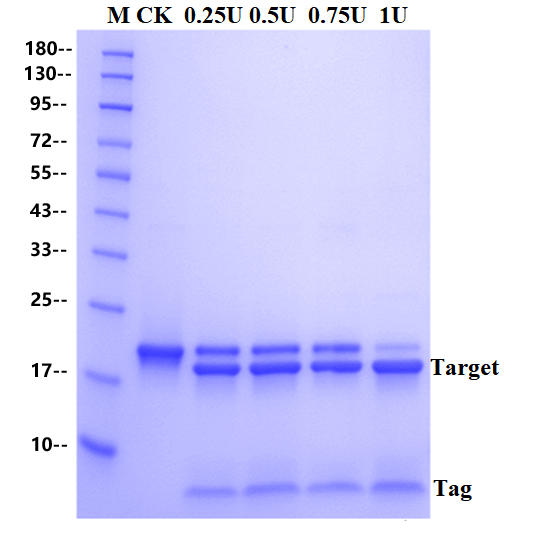
The substrate of 2 μg fusion protein was digested by SUMO Protease at 30 ℃ for 1 hour. The addition amount of SUMO Protease were 0U, 0.25U, 0.5U, 0.75U and 1U, respectively. The substrate is a fusion protein with a molecular weight of 20 KD and digested completely into two bands.
Product Details
Product Details
Product Specification
| Species | Yeast |
| Expression System | E.coli |
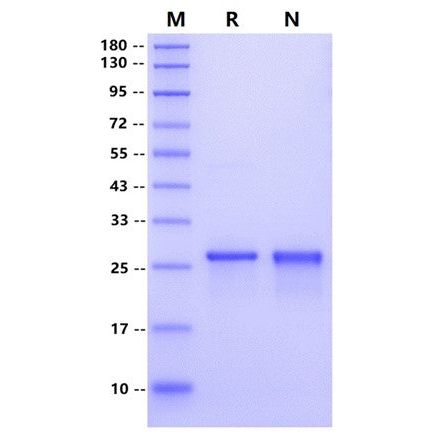
| Molecular Weight | 27 kDa (Reducing) |
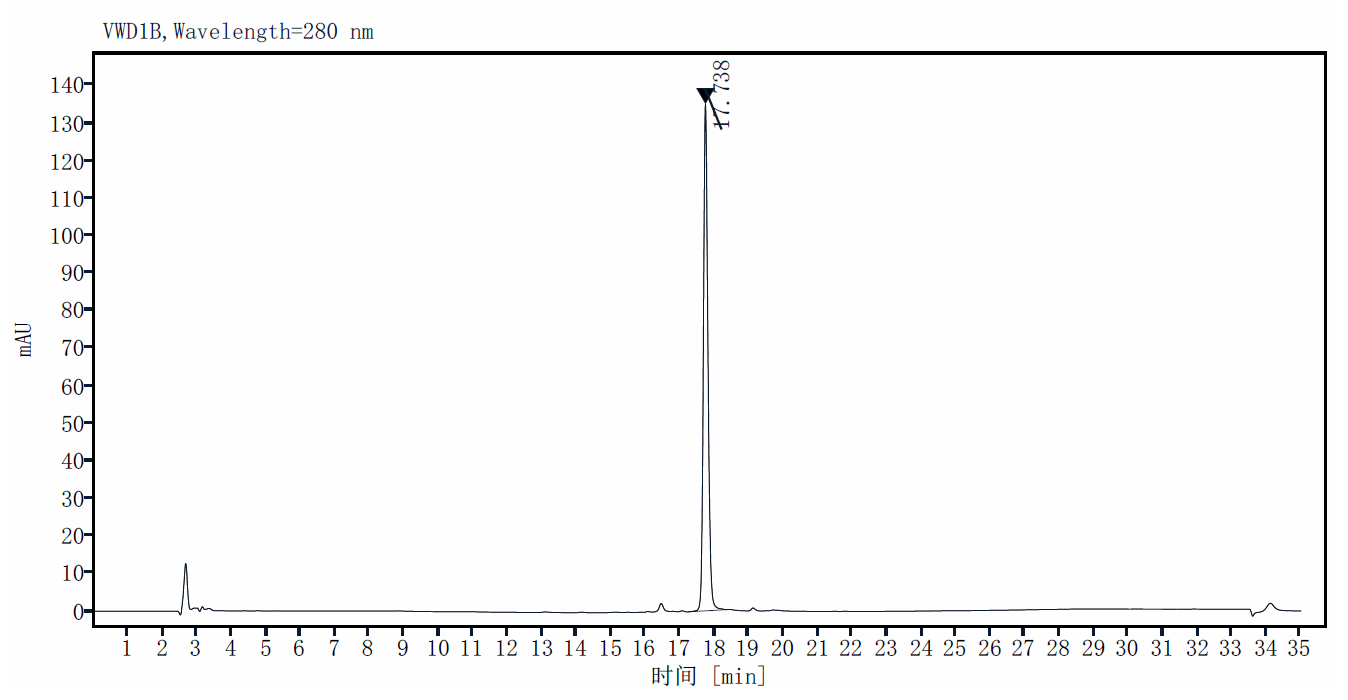
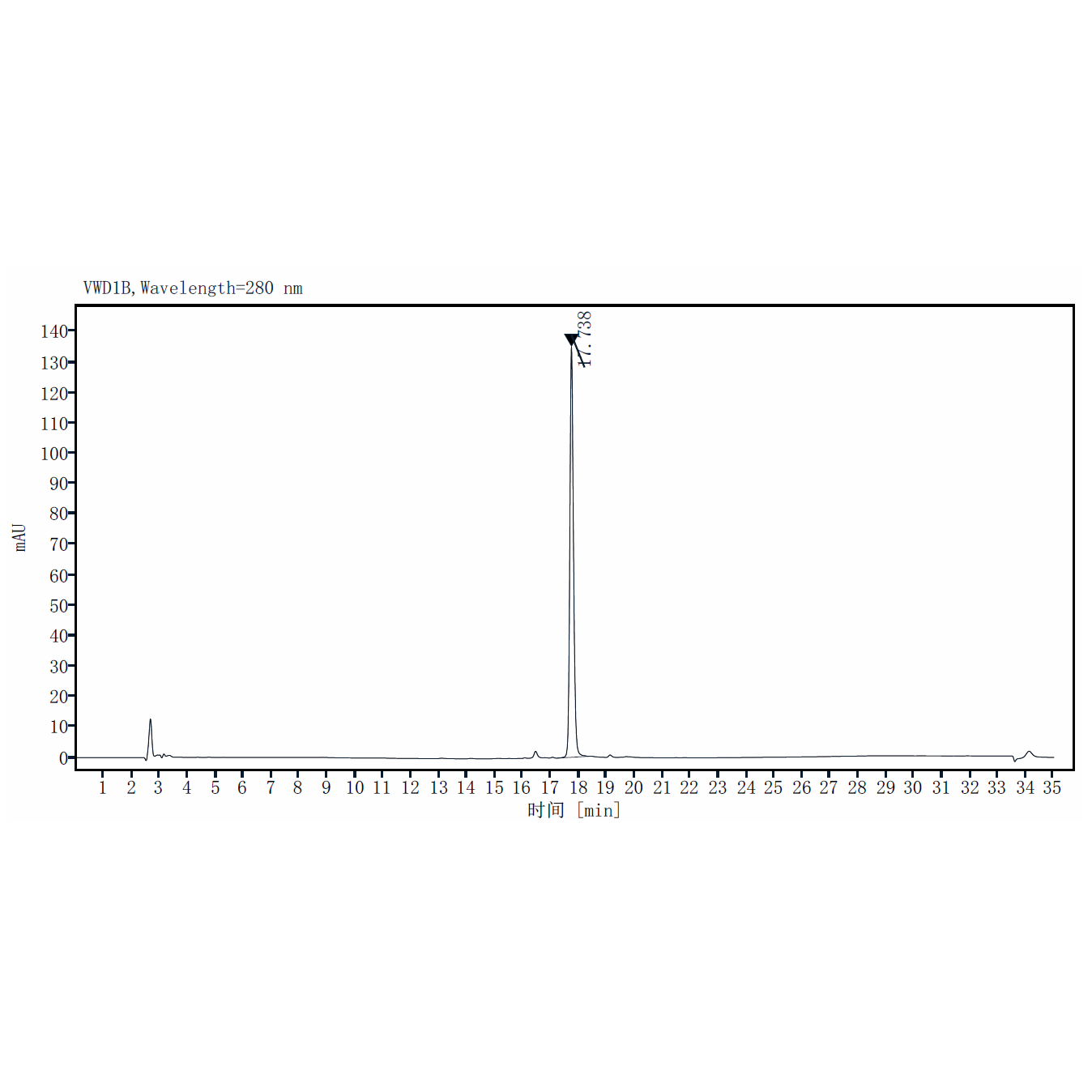
| Purity | >95% by SDS-PAGE and RP-HPLC |
| Endotoxin | <1EU/μg |
| Conjugation | Unconjugated |
| Tag | No Tag |
| Physical Appearance | Liquid |
| Storage Buffer | 20mM PB, pH7.4, 200mM NaCl, 0.1% NP-40, 0.5mM DTT, 50%(v/v) Glycerol |
| Stability & Storage | · 12 months from date of receipt, -20 to -70 °C as supplied. |
Background
Saccharomyces cerevisiae-derived Small ubiquitin-like modifier (SUMO, Smt3) is commonly used as a protein fusion domain to facilitate expression and purification of recombinant proteins, and a Saccharomyces cerevisiae-derived SUMO-specific protease(Ulp1) is then used to remove SUMO tag from these proteins in a ‘scarless’ manner. SUMO Protease cleaves in a highly specific manner, recognizing the tertiary structure of the SUMO tag, rather than an amino acid sequence, and hydrolyzes the peptide bond in the x-Gly-Gly-x sequence after the Gly-Gly bond at the C-terminus of the SUMO tag. The SUMO Protease cleavage proteins over wide ranges of temperature (4℃-30℃), ionic strengths(0-400 mM NaCl) and pH(7.0-9.0), and easily removed from the cleavage reaction by Immobilized Metal Affinity chromatography (IMAC).
Components
1. SUMO Protease;
2. 10X SUMO Protease Buffer + Salt: 500 mM PB, pH 7.4, 2% Igepal (NP-40), 1.5 M NaCl, 10 mM DTT;
3. 10X SUMO Protease Buffer – Salt: 500 mM PB, pH 7.4,
2% Igepal (NP-40), 10 mM DTT;
Protocol
1.Add the following to a microcentrifuge tube:
Fusion Protein |
20μg |
10X SUMO Protease Buffer +/– Salt
|
5μl |
SUMO Protease |
10U |
ddH2O
|
To 50μl |
2. Mix and incubate at 30°C, Remove 5μl aliquots at 1, 2, 4, and 6 hours.
3. Analyze by SDS-PAGE.
- Keep the concentration of Imidazole less then 150mM, or the activity of the SUMO Protease can be adversely affected.
- For most fusion proteins, SUMO Protease functions optimally in a reaction mixture containing 150 mM NaCl; however, conditions may be optimized by varying the NaCl concentration from 100 mM to 300 mM. Remember to take into account the contribution of salt from the enzyme and from your substrate. When setting up your cleavage reaction, use the appropriate 10X SUMO Protease Buffer +/- Salt.
- Researchers need to optimize their specific reaction conditions. As an initial suggestion, 20 units of SUMO protease can be used per 40μg of target protein for 1 hour at 30 °C, or overnight at 2–8 °C. The cleavage efficiency can then be estimated by SDS-PAGE.
Unit Definition
Picture
Picture
Bioactivity
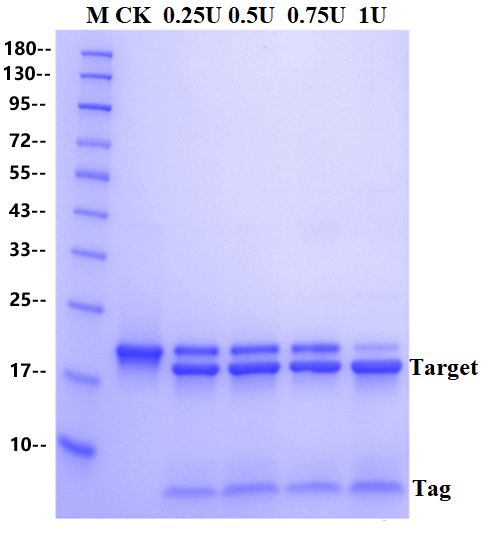
SDS-PAGE
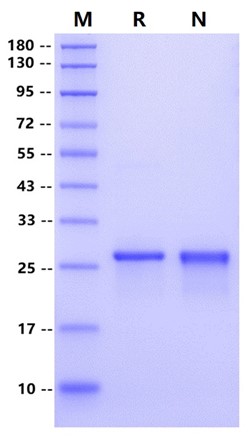
RP-HPLC