M1
Product Details
Product Details
Product Specification
| Species | Mouse |
| Synonyms | Mouse RAW264.7 Cell Polarization Induction Kit II |
Components
|
Contain |
Reference dosage |
S Size |
M Size |
|
IFN-γ Protein, Mouse |
25ng/ml |
65μg |
125μg |
|
Lipopolysaccharide (LPS) solution (500X) |
100ng/ml |
100μl |
100μl*2 |
|
IL-4 Protein, Mouse |
20ng/ml |
50μg |
100μg |
Protocol
Experimental Cell: RAW264.7
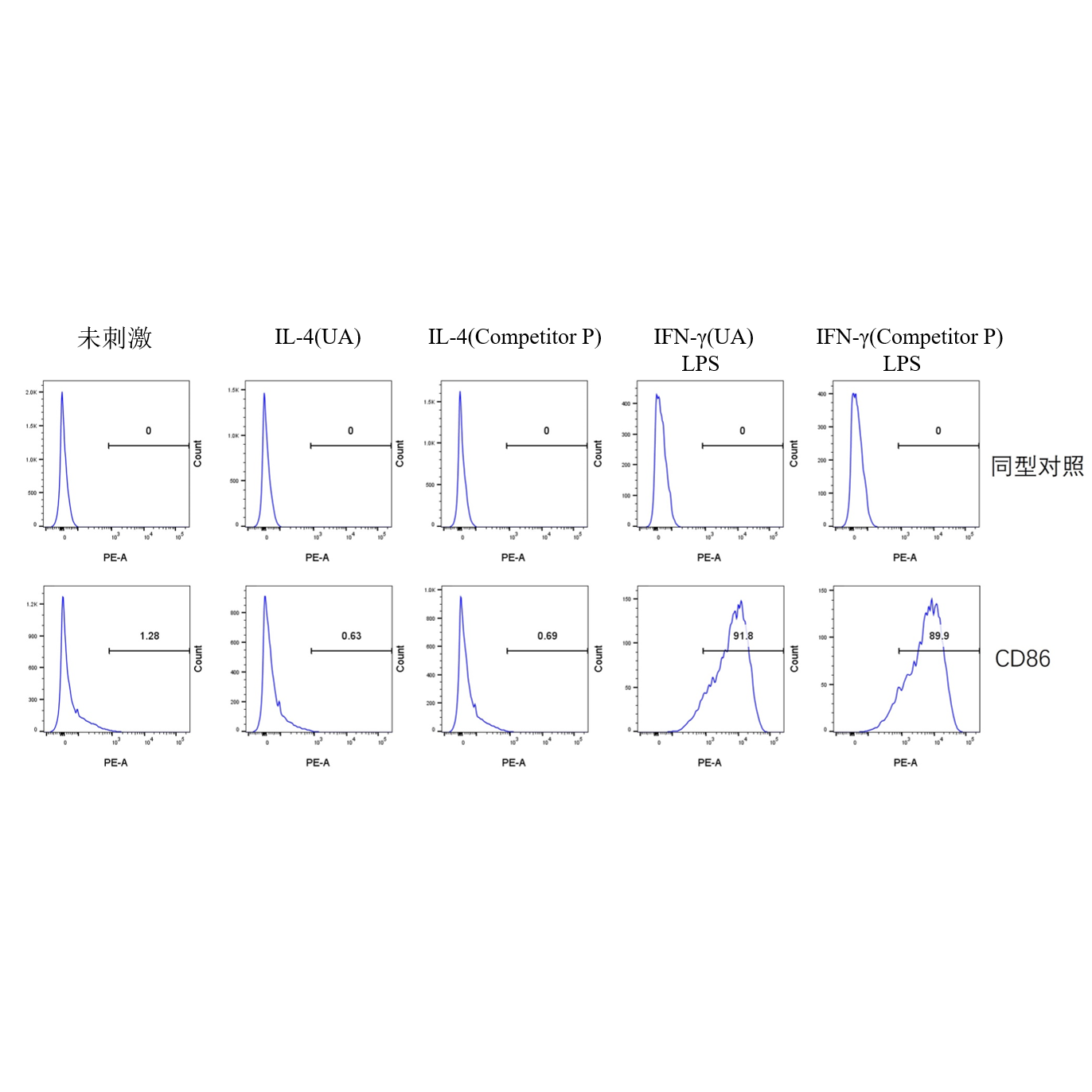
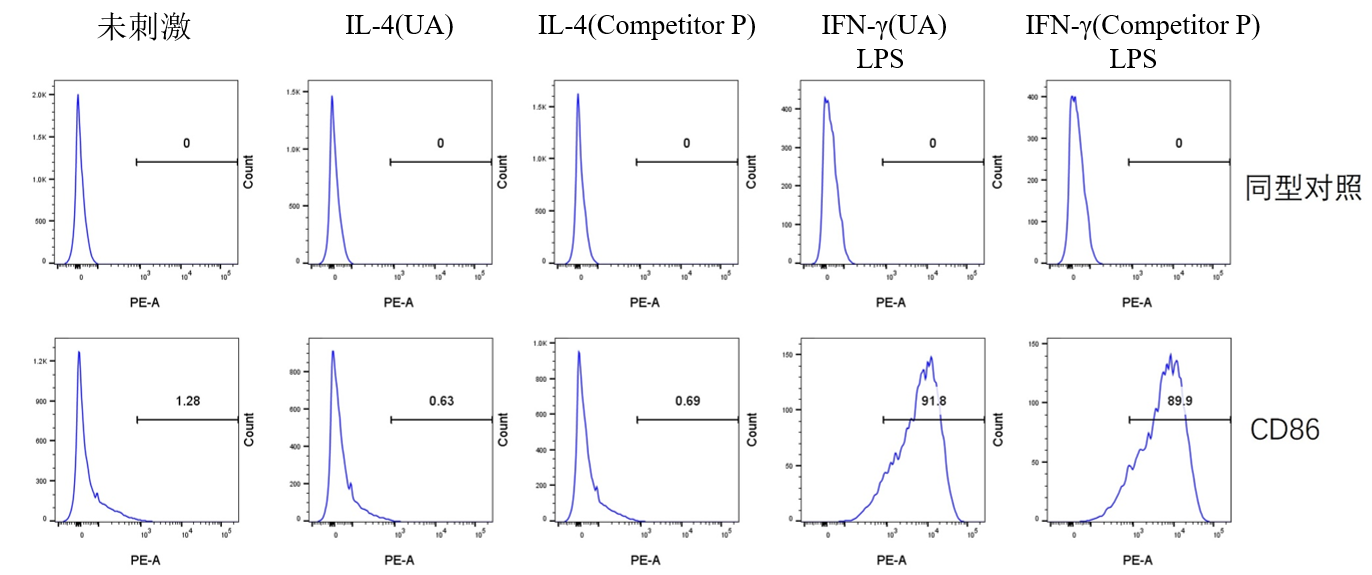
一、M1 Polarization
Culture Conditions: Use a 6-well cell culture plate, with 2×10⁵ cells per well (do not seed too many cells, as this may affect polarization efficiency). Incubate the cells at 37°C with 5% CO₂ for 24 hours. Replace the medium with DMEM supplemented with 2% FBS (to reduce the impact of cytokines in the serum), then add the corresponding cytokines as specified in the table below. Continue incubation at 37°C with 5% CO₂ for another 24 hours.
|
Brand |
Constituent |
Final Conc (ng/ml) |
|
UA |
IFN-γ LPS |
25 100 |
|
Negative control |
Cell Only |
|
Flow Cytometry Detection Procedure:
1.Cell Collection: After 24 hours of cytokine treatment, discard the culture medium supernatant. Wash the cells once with PBS, discard the supernatant, then gently resuspend the adherent 2.cells by pipetting with PBS, and collect the cells by centrifugation.
3.Cell Counting: Resuspend the cells in 0.5 ml of 1% BSA, count the total number of cells, and check the cell viability. The viability should be above 95%.
4.Antibody Incubation: Add PE Rat anti-Mouse CD86 mAb (A27137) (refer to the instruction manual for antibody dosage) or isotype control, and incubate at room temperature for 30 minutes.
5.Cell Washing: Wash the cells with PBS to remove residual antibodies, then resuspend the cells in PBS again.
6.Viability Dye Staining: Add Zombie Violet™ Fixable Viability Kit (423114) to each well (refer to the instruction manual for dosage), and incubate at room temperature in the dark for 15 minutes.
7.Cell Washing: Wash the cells with 1% BSA to remove residual antibodies, then resuspend the cells in 1% BSA.
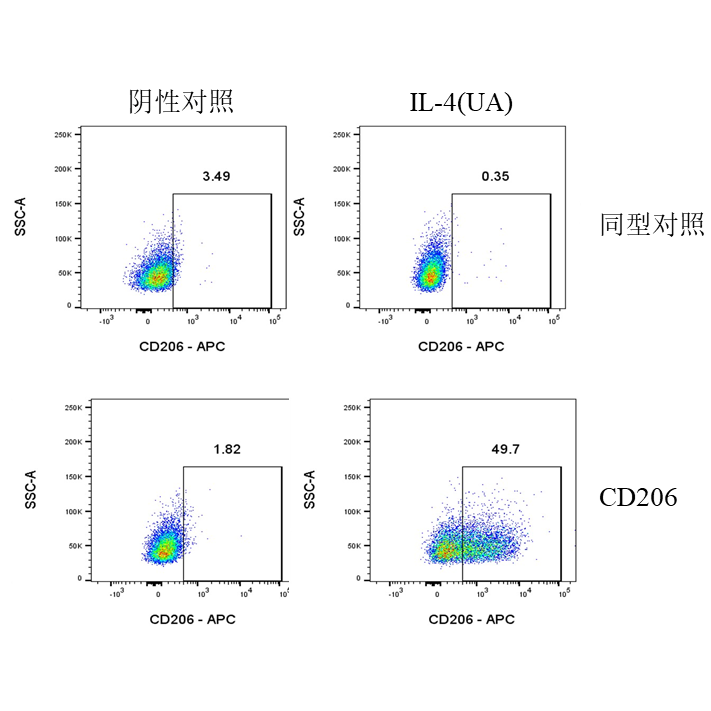
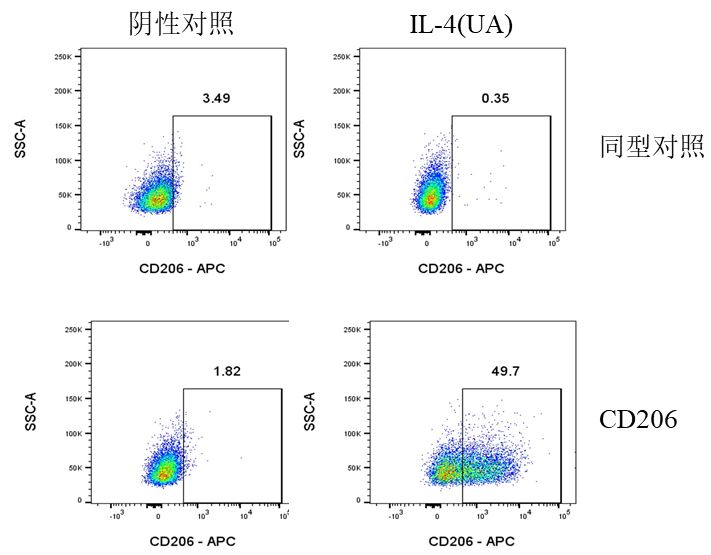
二、 M2 Polarization
Culture conditions: Use a 6-well cell culture plate with 2×10⁵ cells per well (Do not seed too many cells as it may affect polarization efficiency). Incubate at 37°C with 5% CO₂ for 24 hours. Replace the medium with DMEM+2%FBS (to reduce the impact of cytokines in the serum), add corresponding cytokines according to the table below, and incubate at 37°C with 5% CO₂ for another 24 hours.
|
Brand |
Constituent |
Final Conc (ng/ml) |
|
UA |
IL-4 |
20 |
|
Negative control |
Cell only |
|
Flow Cytometry Detection Procedure:
1.Cell Collection: After 24 hours of cytokine intervention, discard the culture medium supernatant. Wash the cells once with PBS, discard the supernatant again, then gently pipette and resuspend the adherent cells with PBS. Collect the cells by centrifugation.
2.Cell Counting: Resuspend the cells in 0.5 ml of 1% BSA. Calculate the total number of cells and check cell viability, which should be above 95%.
Viability Dye Staining: After centrifugation, resuspend the cells with PBS to a density of 1×10^7 cells/ml. Add 100 μl of the cell suspension per well to a 96-well plate or flow cytometry tube, then add the Zombie Violet™ Fixable Viability Kit (423114) (refer to the instruction manual for dosage). Incubate at room temperature in the dark for 15 minutes, centrifuge at 300×g for 5 minutes, and discard the supernatant.
3.Fixation: Resuspend and fix the cells with 0.2 mL of 4% paraformaldehyde. Place at room temperature in the dark for 15 minutes, centrifuge at 300×g for 5 minutes, and discard the supernatant (to remove residual fixative).
4.Membrane Permeabilization: Resuspend and permeabilize the cells with 0.2 ml of 1× Permeabilization Buffer (Thermo #00-8333-56). Place at room temperature in the dark for 30 minutes, centrifuge at 400×g for 5 minutes, and discard the supernatant.
5.Cell Washing: Add 0.2 ml of 1× Permeabilization Buffer per well to resuspend the cells, centrifuge at 400×g for 5 minutes, and discard the supernatant.
6.Blocking: Add 0.1 ml of 1× Permeabilization Buffer per well, and add 2 μg of CD16/CD32 (S0B7003) antibody for blocking. Place on ice for 30 minutes, centrifuge at 400×g for 5 minutes, and discard the supernatant.
7.Antibody Incubation: Add 0.1 ml of 1× Permeabilization Buffer per well, and add either APC anti-Mouse CD206 (MMR) mAb (A26785) (refer to the instruction manual for antibody dosage) or the isotype control (APC Rat IgG Isotype Ctrl Antibody). Incubate at room temperature for 30 minutes, centrifuge at 400×g for 5 minutes, and discard the supernatant (to remove residual antibody).
9.Cell Washing: Add 0.2 ml of 1% BSA per well to wash the cells. Centrifuge at 400×g for 5 minutes, discard the supernatant, and repeat the washing step twice. Resuspend the cells in 200 μl of 1% BSA.
10.Flow Cytometry Analysis: Load the samples onto the flow cytometer for detection.
Picture
Picture
FC
M2