Product Details
Product Details
Product Specification
| Usage |
Need to bring your own test equipment 1. Microplate reader (can measure the absorption value of 450nm detection wavelength and 540nm or 570nm correction wavelength) 2. High precision liquid dispenser and disposable suction head 3. Distilled water or deionized water 4. Washing bottle (spray bottle), multi-channel plate washer or automatic plate washer 5. 500mL cylinder 1, preparation before the experiment 1. Sample collection and storage ① Cell culture supernatant: particles should be removed by centrifugation; Test the samples immediately. If the sample is not tested in time after collection, it is recommended that the sample be divided according to the dosage and stored in the refrigerator at -20 ° C to avoid repeated freezing and thawing. Samples may need to be diluted (1×) Dilute. ② Serum: Samples were collected using a serum separation tube (SST) and samples were left at room temperature for 30 minutes. The samples were centrifuged at 1000g for 15 min. Serum was immediately removed and tested immediately. If the sample is not tested in time after collection, it is recommended to repack according to a single dosage and freeze in ≤ -20℃ refrigerator to avoid repeated freezing and thawing. Samples may need to be diluted (1×) Dilute. ③ Plasma: Plasma was collected using EDTA, heparin, or citric acid as an anticoagulant, centrifuged at 1000g for 15 min within 30 min of collection, and tested immediately. If the samples are not detected in time after collection, it is recommended to separate the samples according to the single dosage and freeze them in &le. -20℃ refrigerator to avoid repeated freezing and thawing. Samples may need to be diluted (1×) Dilute. 2 Reagent preparation (Place all reagents and samples at room temperature for 15 minutes before use. It is recommended that all experimental samples and standards do double hole detection ) 1× Preparation of washing solution: concentrated washing solution in the kit is 20× Mother liquor, diluted to 1× with distilled water before use; Working liquid. Example: Take 10mL concentrated washing solution +190mL distilled water to 200mL, the actual operation can be calculated first, then make up. ②1× Dilution with buffer preparation: concentrate dilution in the kit with buffer 10× Mother liquor, diluted to 1× with distilled water before use; Working solution. example: Take 3mL of concentrated dilution with buffer +27mL of distilled water to a constant volume of 30mL. In practical operation, the required dilution buffer can be calculated according to the dilution multiple of the sample, and then the preparation can be made. ③ Detection of antibody: the dry powder was centrifuged to the bottom of the tube, and 110uL dilution buffer (1×) was used. Dissolve and let stand at room temperature for 5 minutes to obtain 100× Mother liquor; Dilute to 1× before use; Working solution. Calculate the desired volume by using 100uL per well. example: 10 Wells were used, then take 10uL of 100 times the working concentration of the test antibody, using dilution buffer (1×) Constant volume to 1mL, get 1mL of 1× The working concentration of the detected antibody. ④SA-HRP: SA-HRP is 40× Mother liquor, use dilution buffer before use (1×) Dilute to make 1× Working solution, 100uL required per well. example: used 10 holes, then take 25uL of 40× Mother liquor +975uL dilution buffer (1×) Constant volume to 1mL to obtain 1× of 1mL; The working concentration of the detected antibody. ⑤ Chromogenic agent: according to 100uL per well, calculate the amount needed for this test, take out the corresponding volume of chromogenic agent, avoid light; The removed chromogenic agent is only used on the same day. ⑥ Standards: lyophilized standards with dilution buffer (1×) Redissolve, redissolve volume 1000uL, to obtain a concentration of 1000pg/mL standard mother liquor. Gently shake for at least 5 minutes, it is fully dissolved. Add 300uL dilution buffer (1×) to each dilution tube. . The standard mother liquor is diluted according to the picture below, and each tube must be fully mixed before pipetting to the next tube. The standard mother liquor without dilution can be used as the highest point of the standard curve (1000pg/mL), and the dilution buffer (1×) Can be used as the zero point of the standard curve (0pg/mL). 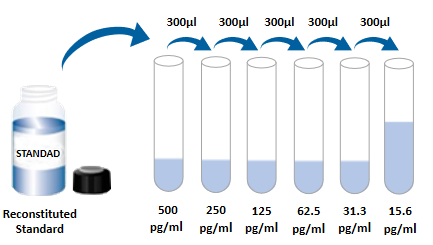 2, operation steps 1. Prepare all required reagents and standards; 2 Remove the microplate from the sealed bag that has been balanced to room temperature, and put the unused slat back into the aluminum foil bag and re-seal it; 3. Add 300uL washing solution to the microplate, let it soak for 30 seconds, discard the washing solution and pat the microplate dry on absorbent paper, please use immediately do not let the microplate dry; 4. Add different concentrations of standard, experimental samples or quality control into the corresponding Wells, 100uL for each well. The Wells were sealed with plate adhesive and incubated at room temperature for 2 hours. 5 Suck the liquid out of the plate and wash the plate using a bottle washer, a multi-channel plate washer, or an automatic plate washer. Add 300uL of washing liquid to each well, and then suck the washing liquid out of the plate. Repeat 3 times. Every time you wash the plate, try to absorb the residual liquid to help you get a good test result. At the end of the last plate wash, please blot all the liquid in the plate or invert the plate and pat all the residual liquid in the absorbent paper; 6. Add 100uL detection antibody to each microwell. Seal the reaction Wells with sealer tape and incubate for 2 hours at room temperature; 7. Repeat the plate washing operation of step 5; 8. Add 100 ULSA-HRP to each microwell and incubate for 20 minutes at room temperature. Be careful to avoid light; 9. Repeat step 5 to wash the plate; 10. Add 100uL of color development solution to each microwell, incubate at room temperature for 5-30 minutes, pay attention to avoid light; 11. Add 50uL of termination solution to each microwell, and the color of the solution in the well will change from blue to yellow. If the color of the solution turns green or the color change is inconsistent, tap the microplate to mix the solution evenly; 12. Within 30 minutes after the termination solution is added, the absorbance value at 450nm is measured using a microplate reader and 540nm or 570nm is set as the correction wavelength. If the dual wavelength correction is not used, the accuracy of the results may be affected; 13 Calculation results: The corrected absorbance values (OD450-OD540/OD570), the compound reading were averaged for each standard and sample, and then the average zero standard OD value was subtracted. Standard curves were created by 4-parameter logic (4-PL) curve fitting using computer software. Alternatively, a curve can be generated by plotting the logarithm of the concentration of the standard against the logarithm of the corresponding OD value, and the best fit line can be determined by regression analysis. This process produces an adequate but less accurate fit to the data. If the sample is diluted, the concentration should be multiplied by the dilution.  3, kit parameter 1. Recovery rate: Different levels of mouse IL-4 were incorporated into the cell culture medium samples and the recovery rate was determined. Recovery range in 75-112%, the average recovery rate at 90%. 2. Sensitivity: The minimum detectable dose (MDD) of IL-4 in mice was generally less than 2.3pg/mL. The lowest detectable value was calculated as the corresponding concentration based on the average of the zero absorbance values of 20 standard curves plus two standard deviations. 3. Correction: the ELISA kit by high purity recombinant e. coli expression correction by IL - 4 protein in mice. 4. Linear: four different samples with high levels of IL - 4 in mice, and then use thinner (1 & times) Linearity was determined by dilutes the samples to the detection range.
5. Specificity: This ELISA can detect native and recombinant mouse IL-4 protein. The following factors in diluent (1 & times) The concentration of the mixture 50 ng/mL to detect and IL - 4 cross reaction in mice. To the interference of 50 ng/mL factor added to the middle scope IL - 4 reference substance in the restructuring of the mice, the interference of IL - 4 to test in mice. No significant cross-reaction or interference was observed.
4, common problem resolution
1. The white board (no color), after the completion of color
2. Flower plate (blank, negative and positive controls were normal, but the OD value of sample Wells was significantly higher)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Theory | Double antibody sandwich enzyme-linked immunosorbent assay was used in this kit. Specific anti-mouse IL-4 antibody was precoated on a high-affinity microplate. The standard substance, test sample and biotinylated detection antibody were added into the well of microplate. After incubation, IL-4 present in the sample combined with solid-phase antibody and detection antibody to form immune complexes. After washing to remove the unbound material, the horseradish peroxidase-labeled Streptavidin-HRP was added. After washing, the chromogenic substrate was added and the color was developed in the dark. The reaction was terminated by adding the termination solution, and the absorbance value was measured at 450nm wavelength (reference correction wavelength 540nm or 570nm). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Composition |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Background | Interleukin-4 (IL-4), also known as B cell stimulating factor 1, is a monomeric cytokine of about 18-20 kda that has pleiotropic effects in immune responses. The precursor of mouse IL-4 is 140 amino acids, including a 20-amino acid signal peptide and a 120-amino acid mature chain. IL-4 contains three N-linked glycosylation potential sites, three intra-chain disulphide bonds, and its structure is a bundled tetra-α-spirochetes. Similar to human IL-4, mouse IL-4 has a small selective splicer. "Unlike human IL-4, the expression of this spliceosome is thought to be low and of little significance." The amino acid sequence of mature mouse IL-4 shared 43% and 63% identity with human and rat IL-4, respectively. Studies have shown that the activities of human, mouse and rat IL-4 are species specific. IL - 4 by toward Th2 CD4 + T cells, basophils and mast cells and NKT cells, gamma delta T cells with eosinophilic cells expressed. IL - 4 has played a key role in type 2 immune reaction, can promote the Th2 differentiation and B cell secretory immunoglobulin G1 and immunoglobulin E subtypes. IL-4 causes Th2 differentiation by binding to type I or type II receptor complexes containing IL-4 receptor alpha subunits coupled to the γ chain or IL13Rα1, respectively. IL - 4 by cell surface receptors of different dimers, activate JAK family of protein complex amino acid kinase, cause its cytoplasm C phosphorylation. This triggers the recruitment and phosphorylation of STAT6. The phosphorylation and conformational changes of STAT6 lead to dimerization, nuclear translocation, binding and transcriptional activation of STAT6 target genes, including IL-4, IL-5, IL-13, and Th2-specific cytokines GATA3 and c-Maf. On the function, IL - 4 B cell proliferation, survival, and promote mice immune globulin class into IgG1 and IgE, promote the mast cells, eosinophils, and basophils start and chemokines, promote Th2 phenotype naive CD4 + cells, and promote the epithelial cell proliferation and activation. IL-4 is also an important cytokine in tumor immunology. In early experiments in mice, the researchers found that IL-4 had potent anti-tumor abilities. Mice reject tumors expressing IL-4 and develop long-term antitumor immunity. This may be due to the antiangiogenic effect of IL-4 or its ability to activate, select for CD8+T cells. "Paradoxically, emerging evidence suggests that IL-4 is a tumor-promoting molecule, and therefore, IL-4 is a reverse-acting cytokine." "A possible explanation is that IL-4 induces upregulation of antiapoptotic molecules in tumor cells and downregulation of cytolytic molecules in CD8+T cells." In addition, tumor-produced IL-4 is also thought to act on local tumor-associated macrophages (TAMs) to induce the secretion of cathepsins that promote cell migration. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Notes | 1. Please use the kit within the validity period. 2. Components of different kits and different batch kits should not be mixed. 3. If the sample value is greater than the highest value of the standard curve, the sample should be diluted with (1×) The samples were diluted and retested. "If the cell culture supernatant sample needs to be diluted in a distributed manner, cell culture medium may be used for intermediate dilutions, except for the last step when diluent is used." 4. The difference of the test results can be caused by a variety of factors, including the operation of the experimenter, the use of the pipettor, the washing technique, the reaction time or temperature, the storage of the kit, etc. 5. The termination solution in the kit is acidic. Please protect your glasses, hands, face and clothes when using it. 6. For scientific research only, not for in vitro diagnosis. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Temp. | Unopened kit, 2-8° C Storage | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Test Range | 15.6pg/mL-1000pg/mL |
Picture
Picture
Immunohistochemistry



