P5 D2
Product Details
Product Details
Product Specification
| Endotoxin | <0.1EU/μg |
| Physical Appearance | Lyophilized powder |
| Reconstitution | Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation. |
| Stability & Storage | ·12 months from date of receipt, lyophilized powder stored at -20 to -80℃. |
Background
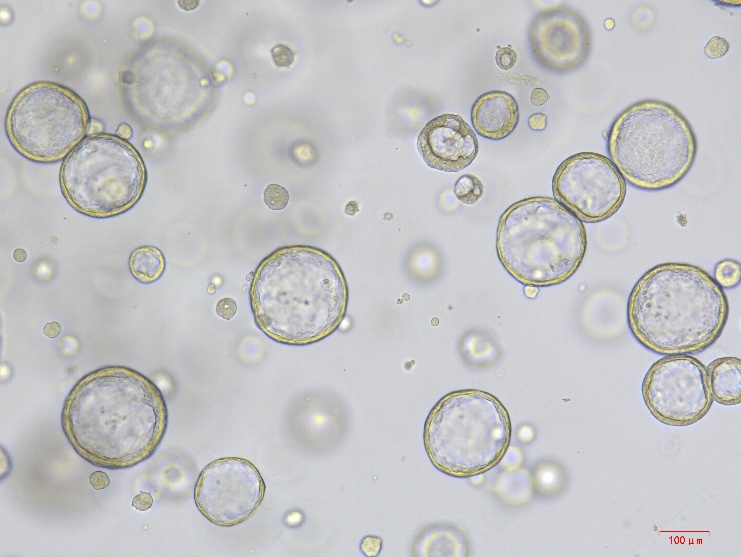
The mouse lung organoid cell factor kit is based on an optimized formulation derived from research, and contains key factors such as Wnt3a and FGF10 that have been verified in the literature. It is specifically designed for the efficient construction of lung organoids and is suitable for studies on development, diseases, and drug screening. Mouse lung organoids are three-dimensional (3D) micro-lung tissue models cultivated in vitro, composed of mouse lung epithelial stem cells (such as basal cells, type II alveolar cells), and have characteristics including the retention of the cell types of the source tissue (such as alveolar epithelial cells, bronchial cells) and some functions (such as surfactant secretion). They simulate lung development, regeneration, and disease processes, compensating for the limitations of traditional 2D cell models and animal models.
Components
Reference dosage |
100ml system |
1000ml system |
|
Cytokines1 |
50ng/ml |
5μg |
50μg |
Cytokines2 |
500ng/ml |
50μg |
500μg |
Cytokines3 |
100ng/ml |
10μg |
100μg |
Cytokines4 |
50ng/ml |
5μg |
50μg |
Cytokines5 |
200ng/ml |
20μg |
200μg |
Cytokines6 |
100ng/ml |
10μg |
100μg |
Cytokines7 |
10ng/ml |
5μg |
10μg |
Cytokines8 |
50ng/ml |
5μg |
50μg |
Protocol
Preparation of Complete Medium for Mouse Lung Organoids: Combine basal medium, cytokines (1-8), and various additives in specified proportions to prepare the complete medium.
1. Primary Culture
(1) Under a biosafety cabinet, extract lung tissue and place it in pre-cooled PBS (supplemented with penicillin, streptomycin, and primary antibiotics) for washing.
(2) Collect the washed tissue fragments into a 1.5 ml EP tube, and mince the tissue as thoroughly as possible (to a homogenate-like consistency) using sterile sharp-pointed ophthalmic scissors. The degree of mincing correlates with digestion time; finer mincing reduces digestion time. Transfer the minced tissue to a 15 ml sterile centrifuge tube.
(3) Add 10 volumes of tissue digestion solution and digest at 37°C with shaking for 10-30 minutes. Monitor the process periodically. Digestion can be terminated when a significant number of cells are observed leaking out under the microscope.
(4) To the confirmed digested tissue suspension, add fetal bovine serum (FBS) to a final concentration of 2-5% or BSA to a final concentration of 0.1%. Mix by pipetting.
(5) Filter the tissue and cell suspension through a 70 µm cell strainer. Centrifuge the filtered cell suspension at 300 g for 3 minutes, then discard the supernatant.
(6) If obvious red precipitate (red blood cells) is observed in the pellet after centrifugation, add 2 ml of red blood cell lysis buffer, mix by pipetting, and incubate for 3 minutes. Centrifuge at 300 g for 3 minutes, then discard the supernatant.
(7) Resuspend the pellet in an appropriate amount of basal medium or PBS.
(8) Mix Matrigel and cells at a suitable ratio. For a 24-well cell culture plate, plate 25-30 µl of the Matrigel-cell mixture per well (perform operations at 4°C).
(9) Place the prepared culture plate in a 37°C incubator for 20-30 minutes for gelation. Add an appropriate amount of complete medium for mouse lung organoids to initiate culture.
2. Organoid Passaging
(1) Aspirate the medium using a pipette and add 1-2 ml of 4°C PBS to each well. Incubate for 2 minutes.
(2) Gently disrupt the Matrigel by pipetting and collect the contents into a 15 ml centrifuge tube. Adjust the volume to 10-14 ml with PBS and let it stand at 4°C for 20-30 minutes (grouping every 3-5 wells as a set). Centrifuge at 300 g for 3 minutes, discard the supernatant, and retain the pellet.
(3) Add 1 ml of organoid digestion solution to the collected pellet, mix by pipetting, and incubate at 37°C for 2-3 minutes for digestion. Terminate digestion by adding basal medium. Centrifuge at 300 g for 3 minutes, discard the supernatant, and retain the pellet.
(4) Resuspend the organoids in an appropriate amount of Matrigel. Plate 25-30 µl of the Matrigel-organoid mixture per well in a 24-well cell culture plate. Place the plate in the incubator for 20-30 minutes for gelation, then add an appropriate amount of complete medium for mouse lung organoids to continue culture.
3. Organoid Cryopreservation
(1) Aspirate the medium using a pipette and add 1-2 ml of 4°C PBS to each well. Incubate for 2 minutes.
(2) Gently disrupt the Matrigel by pipetting and collect the contents into a 15 ml centrifuge tube. Adjust the volume to 10-14 ml with PBS and let it stand at 4°C for 20-30 minutes (grouping every 3-5 wells as a set). Centrifuge at 300 g for 3 minutes, discard the supernatant, and retain the pellet.
(3) Add an appropriate amount of organoid freezing medium, gently resuspend by pipetting. For a 24-well plate, cryopreserve the pellet from 2-3 wells in one cryovial with a volume of 1 ml per vial.
(4) Label the vials clearly, perform programmed freezing, and then transfer them to liquid nitrogen for long-term storage.
4. Organoid Thawing
(1) Pre-aliquot 10 ml of DMEM/F12 basal medium into a 15 ml centrifuge tube.
(2) Retrieve the cryopreserved organoid vial from the liquid nitrogen tank and quickly thaw it in a 37°C water bath.
(3) During thawing, gently agitate the cryovial to ensure the freezing medium is completely dissolved within 1-2 minutes.
(4) Quickly transfer the thawed organoids to the 15 ml centrifuge tube containing the pre-warmed basal medium. Gently pipette 6-8 times. Centrifuge at 300 g for 3 minutes, then remove the supernatant and collect the organoid pellet.
(5) Resuspend the pellet in Matrigel. Plate 25-30 µl of the Matrigel-organoid mixture per well in a 24-well cell culture plate. Place the plate in the incubator for 20-30 minutes for gelation, then add an appropriate amount of complete medium for mouse lung organoids to initiate culture.
Guidelines
Avoid vortex oscillation; Store in aliquots to reduce the number of freeze-thaw cycles
Picture
Picture
Bioactivity