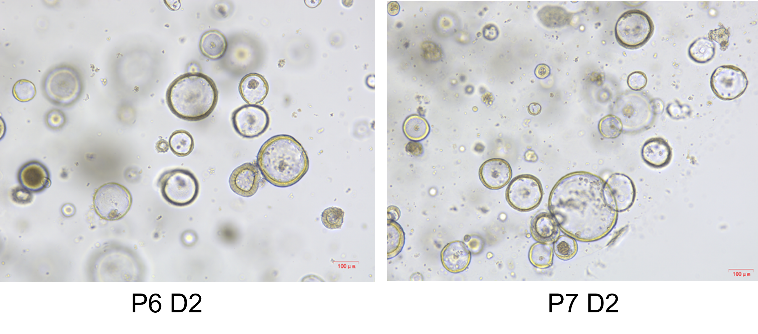
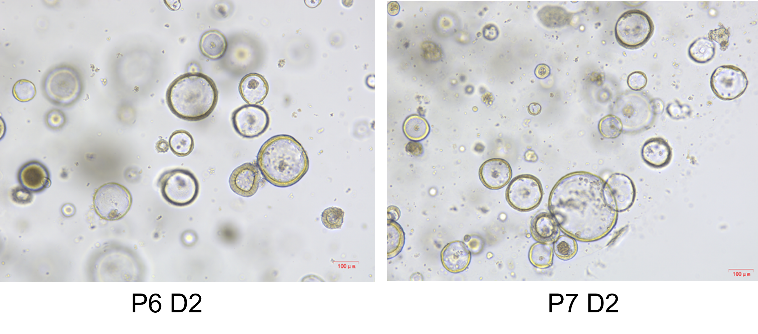
Product Details
Product Details
Product Specification
| Physical Appearance | Lyophilized Powder |
| Reconstitution | Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation. |
| Stability & Storage | -20℃ |
Components
Reference dosage |
Reference dosage |
100ml system |
1000ml system |
Cytokines1 |
50ng/ml |
5μg |
50μg |
Cytokines2 |
500ng/ml |
50μg |
500μg |
Cytokines3 |
20ng/ml |
5ug |
20ug |
Cytokines4 |
250ng/ml |
25μg |
250μg |
Protocol
1. Primary Culture
(1) In a biosafety cabinet, completely dissect the mouse colon tissue and place it in pre-cooled PBS (supplemented with penicillin, streptomycin, and primary antibiotics). Using sterile scissors, cut the intestinal segment open longitudinally and lay it flat with the lumen facing upwards. Use a glass coverslip to gently scrape off intestinal debris and residues 2-3 times back and forth. Rinse the colon with pre-cooled PBS.
(2) Hold one end of the colon with forceps and cut it into small segments, approximately 3-5 mm in size. Collect and transfer the segments into a 50 ml sterile centrifuge tube. Add pre-cooled PBS and wash 2-3 times.
(3) Add 30 ml of 5 mM EDTA/PBS solution (30 ml PBS + 300 µl 0.5 M EDTA) to the centrifuge tube. Place the colon tissue into the tube for digestion. Digest on a shaking platform at 4°C for about 30 minutes, periodically checking under a microscope. The detachment of crypts indicates the endpoint of digestion.
(4) Discard the digestion solution. Add pre-cooled PBS and gently swirl to remove EDTA.
(5) Add 30 ml of pre-cooled PBS containing 0.1% BSA. Vortex to dislodge the crypts from the colon tissue.
(6) Keep the supernatant. Pass it through a 100 µm cell strainer. Evenly distribute the filtered cell suspension into two 15 ml centrifuge tubes. Centrifuge at 1000 rpm for 5 minutes. After centrifugation, discard the supernatant.
(7) Repeat steps 5-6 twice to increase the yield of crypts.
(8) Resuspend the pellet in an appropriate amount of basal medium or PBS.
(9) Mix the basement membrane matrix (e.g., Matrigel) with the crypts at an appropriate ratio. For a 24-well cell culture plate, plate 25-30 µl of the matrix/crypt mixture per well.
(10) Place the prepared culture plate in a 37°C incubator for 20-30 minutes to allow the matrix to solidify. Add an appropriate amount of pre-warmed (room temperature) complete mouse colon organoid medium to begin culture.
2. Organoid Passaging
(1) Aspirate the culture medium using a pipette. Add 1-2 ml of 4°C PBS to each well and incubate for 2 minutes.
(2) Gently pipette to disrupt the solidified matrix. Collect the contents into a 15 ml centrifuge tube. Adjust the volume to 10-14 ml with PBS. Incubate at 4°C for 20-30 minutes to dissolve the matrix (pool contents from every 3-5 wells into one tube). Centrifuge at 1000 rpm for 5 minutes. Discard the supernatant and retain the pellet.
(3) Add 1 ml of organoid digestion solution to the collected pellet. Pipette to mix. Digest at 37°C for 2-5 minutes. Add DMEM/F12 basal medium to stop the digestion. Centrifuge at 1000 rpm for 5 minutes. Discard the supernatant and retain the pellet.
(4) Resuspend the organoids in an appropriate amount of basement membrane matrix. Plate 25-30 µl of the matrix/organoid mixture per well in a 24-well cell culture plate. Place the plate in the incubator for 20-30 minutes for the matrix to solidify. Add an appropriate amount of complete mouse colon organoid medium.
3. Organoid Cryopreservation
(1) Aspirate the culture medium using a pipette. Add 1-2 ml of 4°C PBS to each well and incubate for 2 minutes.
(2) Gently pipette to disrupt the solidified matrix. Collect the contents into a 15 ml centrifuge tube. Adjust the volume to 10-14 ml with PBS. Incubate at 4°C for 20-30 minutes to dissolve the matrix (pool contents from every 3-5 wells into one tube). Centrifuge at 1000 rpm for 5 minutes. Discard the supernatant and retain the pellet.
(3) Add an appropriate amount of organoid freezing medium. Gently pipette to resuspend the pellet. For a 24-well plate scale, cryopreserve the organoids from 2-3 wells in one cryovial (1 ml volume per vial).
(4) Label the cryovials appropriately. Perform controlled-rate freezing, then transfer the vials to liquid nitrogen for long-term storage.
4. Organoid Thawing
(1) Pre-aliquot 10 ml of DMEM/F12 basal medium into a 15 ml centrifuge tube.
(2) Retrieve the cryopreserved organoid vial from the liquid nitrogen tank. Quickly thaw it in a 37°C water bath.
(3) During thawing, gently agitate the cryovial to ensure complete melting of the freezing medium within 1-2 minutes.
(4) Quickly transfer the thawed organoid suspension into the 15 ml tube containing the basal medium. Gently pipette 6-8 times to mix. Centrifuge at 1000 rpm for 5 minutes. Aspirate the supernatant and collect the organoid pellet.
(5) Resuspend the pellet in basement membrane matrix. Plate 25-30 µl of the matrix/organoid mixture per well in a 24-well cell culture plate. Place the plate in the incubator for 20-30 minutes for the matrix to solidify. Add an appropriate amount of complete mouse colon organoid medium.
Guidelines
Avoid vortex oscillation; store in separate portions to reduce the number of freeze-thaw cycles.
Picture
Picture
Bioactivity