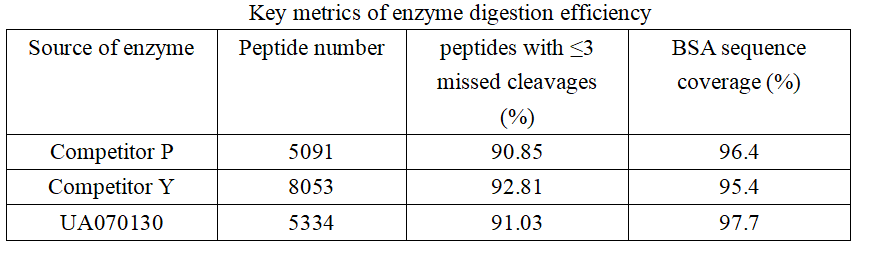
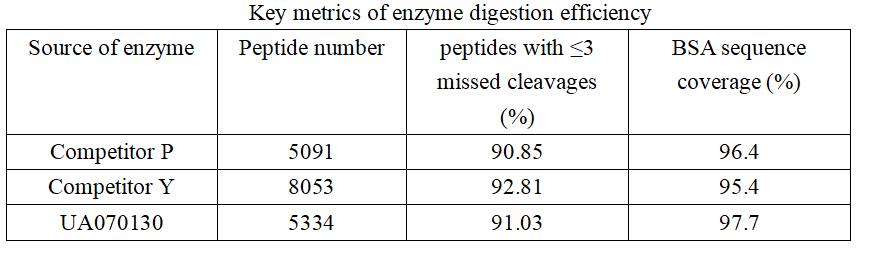
In terms of sequence coverage, UA070130 demonstrates a slight advantage, with enzyme P and enzyme Y exhibiting coverage values that are 1.3% and 2.3% lower, respectively. However, when considering peptides with no more than three missed cleavage sites, a different trend emerges. Enzyme Y yields the highest proportion, followed by enzymes UA070130 and P, which show marginally lower values. These results indicate that the three enzymes display comparable efficacy in protein digestion.
Product Details
Product Details
Product Specification
| Synonyms | Trypsin(pig); porcine pancreas; Trypsin,Mass Spectrometry Grade |
| Molecular Weight | 24±2 kDa (Reducing) |
| Purity | >95% by SDS-PAGE |
| Tag | No Tag |
| Physical Appearance | Lyophilized powder |
| Storage Buffer | 2 mM HCl, 2 mM CaCl2 |
| Reconstitution | Trypsin, Mass Spectrometry Grade should be reconstituted by the addition of 20–200 μL of 2 mM HCl. |
| Stability & Storage | Store at -25 ~ -15℃ for 2 years |
| Reference | Olsen, J. V. Trypsin Cleaves Exclusively C-terminal to Arginine and Lysine Residues[J]. Molecular & Cellular Proteomics, 2004, 3(6):608-614. |
Background
Trypsin is a serine protease commonly found in the vertebrate digestive system that hydrolyzes proteins. Active trypsin specifically hydrolyzes peptide bonds on the carboxyl side of lysine or arginine residues, provided the next residue is not proline. This specificity makes trypsin indispensable for the initial digestion of dietary proteins into smaller peptides, which are then further degraded by other proteases. Trypsin consists of two subunits, the alpha subunit (with 2 polypeptide chains forming it) and the beta subunit (with 1 polypeptide chain forming it). Trypsin, Mass Spectrometry Grade was subjected to reductive methylation, producing a highly active, stable molecule that strongly prevented its autolytic activity and reduced interference from excess peptide fragments generated by self-hydrolysis. It can be widely applied in laboratory proteomics analyses such as protein and peptide sequencing identification and HPLC peptide-map analysis.
Components
Trypsin,Mass Spectrometry Grade lyophilized
2*Reaction buffer:,100 mM Tris-HCl, 40 mM CaCl2, pH 8.0 @ 25°C
Protocol
1 Trypsin, Mass Spectrometry Grade should be reconstituted by the addition of 20–200 μl of 2 mM HCl
2 Set-up a typical reaction as follows
1)Add the following components in sequence
Components |
Volume |
Substrate protein |
5 μL (about 2 ug) |
ddH2O |
4.5 μL |
2*Reaction buffer |
10 μL |
100 ng/μL Tyrpsin |
0.5 μL |
2)Incubate at 37°C for 2~4 h
Guidelines
1. Substrate must be in phosphate-free buffer to prevent calcium precipitation with both reconstituted enzyme and enzyme buffer.
2. Storage buffer: 2 mM HCl, 2 mM CaCl2.
Unit Definition
Picture
Picture
Bioactivity
SDS-PAGE
2μg (R: reducing condition, N: non-reducing condition).
RP-HPLC