Product Details
Product Details
Inspection principle:
Oxidized nicotinamide adenine dinucleotide (NAD +) and reduced nicotinamide adenine dinucleotide (NADH) are coenzymes that transfer electrons during redox reactions, and can be used as cofactors of many enzymes to participate in intracellular reactions.
Determination of total NAD + and NADH:
Ethanol produces acetaldehyde under the action of enzymes. During this reaction, NAD + is converted into NADH. NADH reduces WST-8 to produce an orange-yellow substance under the action of electron coupling reagent, with a maximum absorption peak around 450 nm. The amount of NAD + and NADH in the reaction sample of orange-yellow substance generated in the reaction system.
The amount of NADH was determined alone:
After the sample is extracted and heated in a water bath at 60 °C for 30 minutes, the NAD + in the sample will decompose and only NADH will be retained. NADH reduces WST-8 to produce a yellow substance, and the amount of NADH in the sample can be determined separately.
Determination of NAD + and NAD +/NADH ratios:
According to the total amount of NAD + and NADH obtained in the first two steps and the amount of NADH alone, the amount of NAD + in the sample and the ratio of NAD + to NADH can be obtained. During the above assay, due to the specificity of the enzyme reagent, NADP + and NADPH have no influence on the assay results.
When testing samples with this kit, it is necessary to measure the total protein concentration. It is recommended to use our company's BCA kit (abs9232) for measurement.
Product composition:
| serial number | Name | Specifications | Save |
| Reagent 1 | Extract Extracting Solution | 60 mL × 2 | -20℃ |
| Reagent 2 (Reagent 2) | Buffer Buffer Solution | 16 mL × 1 | -20℃ |
| Reagent 3 | Color developer (Chromogenic Agent) | 5 mL × 1 | -20 ℃, protected from light |
| Reagent 4 (Reagent 4) | Enzyme reagent Enzyme Reagent | Powder × 2 | -20 ℃, protected from light |
| Reagent 5 | Standard | Powder × 2 | -20 ℃, protected from light |
| 96-well plate | 1 plate | ||
| 96-well coating | 2 sheets | ||
| Sample Location Marker Table | 1 sheet |
Note: The reagents are stored strictly according to the storage conditions in the above table, and the reagents in different test kits cannot be mixed. For reagents with smaller volumes, please centrifuge before use, so as not to measure enough reagents. When this kit only measures the total amount of NAD + and NADH or the amount of NADH in a sample, one kit can measure 80 samples; When determining NAD + or the ratio of NAD + to NADH, one kit can determine 40 samples.
Instrument: microplate reader (450 nm), thermostatic water bath, 37 °C thermostatic box
Reagent: PBS (0.01 M, pH 7.4)
Consumables: 10 KD ultrafiltration tube
2. Reagent preparation:
(1) Before testing, all reagents are equilibrated to room temperature.
(2) Preparation of reagent 4 working solution:
Each reagent powder is fully dissolved in 200μL of double-distilled water, placed at 4 °C in the dark for 5 hours before use, and the unused part can be stored at 4 °C in the dark for 7 days.
(3) Preparation of reaction working solution:
Mix the working solution of reagent 4 and reagent 2 at a volume ratio of 1:39, prepare it as needed before use, prepare it for use now, and store it in the dark from light. The prepared working solution is effective within 2 hours.
(4) Preparation of 250μmol/L standard stock solution:
Each reagent 5 is fully dissolved with 200μL of double-distilled water, and the unused part can be stored in the dark for 7 days at-20 °C.
(5) Preparation of 5μmol/L standard solution:
Dilute according to the ratio of 250μmol/L standard stock solution: reagent-volume ratio of 1:49 to obtain 5μmol/L standard, which is prepared and used as needed before use, stored in the dark from light, and used within 1 day.
(7) Dilution of different concentration standards:
| serial number | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
| Standard Concentration (μmol/L) | 0 | 1 | 1.5 | 2 | 2.5 | 3.5 | 4 | 5 |
| 5 μmol/L standard (μL) | 0 | 40 | 60 | 80 | 100 | 140 | 160 | 200 |
| Reagent One (μL) | 200 | 160 | 140 | 120 | 100 | 60 | 40 | 0 |
1) Sample processing
Sample homogenate:
Tissue sample: The homogenate medium is reagent 1, centrifuge at 10000 × g at 4 °C for 10 minutes, take the supernatant, and retain part of the supernatant for protein concentration determination.
Cell samples: After washing the cells with pre-cooled PBS (0.1 M, pH 7.4) on ice, take about 1.5 × 106 cells, add 0.4 mL of pre-cooled reagent-homogenate, centrifuge at 12000 × g for 10 minutes at 4 °C, take the supernatant, and retain part of the supernatant for protein concentration determination.
Sample ultrafiltration:
The homogenates of tissues and cells contain enzymes that can decompose NAD +. It is recommended that after sample extraction and centrifugation, the supernatant should be centrifuged in a 10 KD ultrafiltration tube at 4 °C and 10000 × g for 10 minutes to remove the decomposing enzymes.
When measuring the total amount of NAD + and NADH, the supernatant of the sample to be tested after filtration by ultrafiltration tube is taken for direct measurement.
If you want to measure the amount of NADH separately, take an appropriate amount of the sample to be measured and put the filtrate after ultrafiltration in an EP tube, take it in a water bath at 60 °C for 30 minutes, cool with running water and mix it well to be measured.
2) Sample dilution
Before formal testing, 2-3 samples with large expected differences need to be selected to dilute into different concentrations for pre-experiment. According to the results of pre-experiments, combined with the linear range of this kit: 0.02-5.0 μmol/L, please refer to the following table for dilution (for reference only):
| Sample | Dilution factor | Sample | Dilution factor |
| 10% mouse muscle | Not diluted | 293T cells | Not diluted |
| 10% mouse kidney tissue | Not diluted | Hela cells | Not diluted |
4. Key points of the experiment:
1. After each reagent powder is fully dissolved in 200μL of double-distilled water, it should be placed at 4 °C in the dark for 5 hours before use. Pay attention to preparation in advance.
2. Try to use fresh samples for measurement. After freezing the samples, the measurement results will be reduced or the values will not be measured.
3. The sample extract liquid is heated in a 60 °C water bath for 30 minutes to decompose NAD +. During this process, the EP tube must be sealed to prevent the liquid from volatilizing; After the heating is completed, due to the condensation of water vapor, it needs to be fully mixed before the next operation is carried out.
5. Operation steps:
1. Measurement well: Take 20μL of the sample to be tested and add it to the measurement well corresponding to the enzyme plate.
Standard wells: Take 20μL of different concentration standards and add them to the corresponding standard wells of the enzyme label plate.
2. Take 120μL of the reaction working solution and add it to the measurement well and standard well in step ①.
3. Add 40μL of reagent three to the measurement well and standard well in ②.
4. After vibrating the plate for 5 s and accurately incubating in a 37 °C incubator for 30 min, the OD value of each well was measured at a wavelength of 450 nm with a microplate reader.
6. Operation table:
| Standard hole | Measurement well | |
| Sample to be tested (μL) | — | 20 |
| Different concentration standards (μL) | 20 | — |
| Reaction working solution (μL) | 120 | 120 |
| Reagent III (μL) | 40 | 40 |
| Vibrate the plate for 5 s, accurately incubate in a 37 °C incubator for 30 min, and measure the OD value of each well with a microplate reader at a wavelength of 450 nm. | ||
7. Result calculation:
Standard fit curve: y = ax + b
Formula for calculating the total amount of NAD + and NADH in the sample:
NADtotal (μmol/gprot) = (ΔA-b) ÷ a × f ÷ Cpr
Formula for calculating NADH content in the sample:
NADH (μmol/gprot) = (ΔA-b) ÷ a × f ÷ Cpr
Calculation formula of NAD + content in sample:
NAD + (μmol/gprot) = NADtotal-NADH
Formula for calculating the ratio of NAD + to NADH in the sample:
NAD + ⁄ NADH = (NADtotal-NADH)/NADH × 100%
Notes:
y: standard OD value − blank OD value (OD value when the standard concentration is 0)
x: Concentration of standard
A: slope of the standard curve
b: Intercept of the standard curve
∆ A: measurement well OD value − blank well OD value (OD value when the standard concentration is 0)
Cpr: supernatant protein concentration before ultrafiltration tube filtration (gprot/L)
f: Dilution factor before sample is added to test system
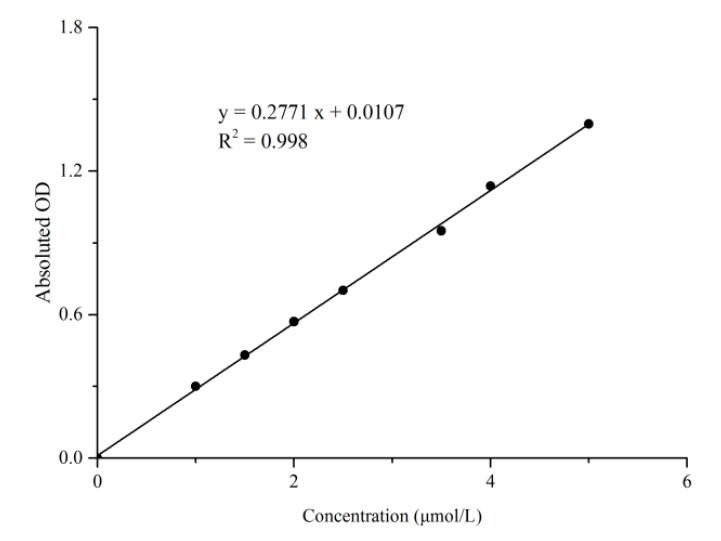
8. Standard curve (data is for reference only)
1. Add 20μL of standard substances with different concentrations, and carry out the experiment according to the operation steps. The OD value of each tube is shown in the following table:
| Standard Concentration (μmol/L) | 0 | 1 | 1.5 | 2 | 2.5 | 3.5 | 4 | 5 |
| OD Value | 0.148 | 0.451 | 0.571 | 0.728 | 0.843 | 1.038 | 1.290 | 1.576 |
| 0.158 | 0.456 | 0.579 | 0.720 | 0.866 | 1.168 | 1.291 | 1.525 | |
| Average OD value | 0.153 | 0.454 | 0.584 | 0.724 | 0.854 | 1.103 | 1.290 | 1.550 |
| Absolute OD value | 0.000 | 0.301 | 0.431 | 0.571 | 0.950 | 1.137 | 1.397 |