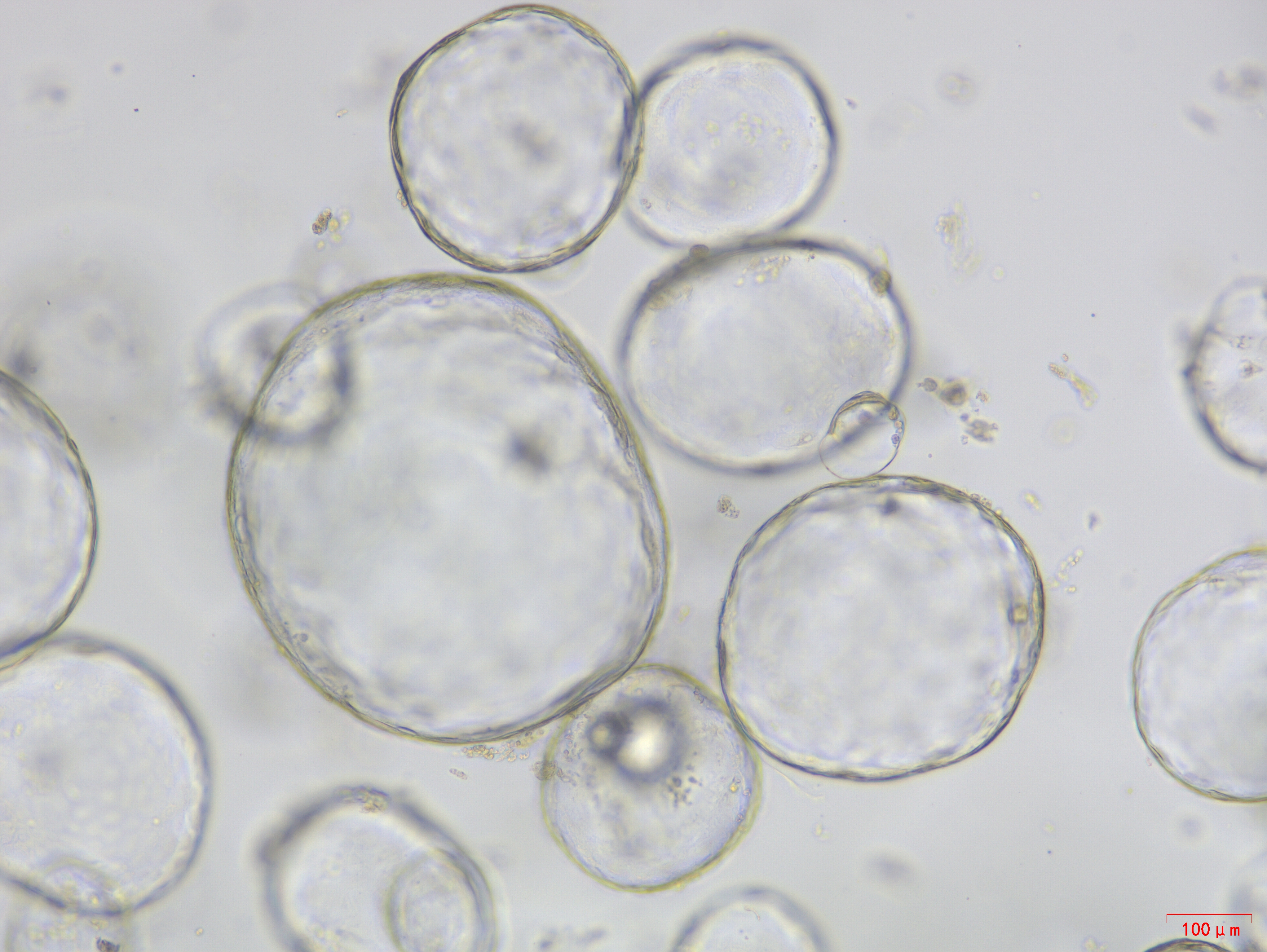
P4 D3
Product Details
Product Details
Product Specification
| Species | Mouse |
| Endotoxin | <0.1EU/μg |
| Physical Appearance | Lyophilized powder |
| Reconstitution | Reconstitute at 0.1-1 mg/ml according to the size in ultrapure water after rapid centrifugation. |
| Stability & Storage | ·12 months from date of receipt, lyophilized powder stored at -20 to -80℃. |
Components
Protein |
Reference dosage |
100ml system |
1000m system |
Cytokines 1 |
50ng/ml |
5μg |
50μg |
Cytokines 2 |
250ng/ml |
25μg |
250μg |
Cytokines 3 |
50ng/ml |
5μg |
50μg |
Cytokines 4 |
100ng/ml |
10μg |
100μg |
Cytokines 5 |
25ng/ml |
5μg |
25μg |
Protocol
Preparation of Complete Medium (Expansion) for Mouse Cholangiocyte Organoids:
Mix the basal medium, cytokines (1-5), and various supplements in specified proportions to prepare the complete medium.
1. Primary Culture
(1) Quickly dissect the abdominal cavity of the mouse in a biosafety cabinet, extract the liver tissue, and rinse it in pre-cooled PBS (supplemented with penicillin, streptomycin, and primary antibiotics).
(2) Collect the rinsed tissue fragments into a 1.5 ml EP tube, and mince the tissue as thoroughly as possible (to a homogenate-like consistency) using sterile sharp-tipped ophthalmic surgical scissors. The degree of mincing affects digestion time; finer mincing shortens digestion time. Transfer the minced tissue to a 15 ml sterile centrifuge tube.
(3) Add 10 volumes of tissue digestion solution and digest at 37°C with shaking for 10–30 minutes. Monitor the process closely. Digestion can be terminated when a large number of cells are released and most cells appear as clusters (Note: Avoid digesting into a single-cell state).
(4) After confirming digestion, add fetal bovine serum (FBS) to a final concentration of 2–5% or BSA to a final concentration of 0.1%, and mix by pipetting.
(5) Filter the tissue and cell suspension through a 70 µm cell strainer. Centrifuge the filtered cell suspension at 1000 rpm for 5 minutes, and discard the supernatant.
(6) If obvious red precipitate (red blood cells) is observed in the pellet after centrifugation, add 2 ml of red blood cell lysis buffer, mix by pipetting, and incubate for 3 minutes. Centrifuge at 1000 rpm for 5 minutes and discard the supernatant.
(7) Resuspend the pellet in an appropriate amount of basal medium or PBS.
(8) Mix the basement membrane matrix (e.g., Matrigel) and cells at a suitable ratio. For a 24-well cell culture plate, plate 25–30 µl of the matrix-cell mixture per well (perform操作 at 4°C).
(9) Place the plate in a 37°C incubator for 20–30 minutes to allow gelation. Add an appropriate amount of complete medium (expansion) for mouse cholangiocyte organoids for culture.
2. Passaging of Organoids
(1) Aspirate the medium using a pipette, add 1–2 ml of 4°C PBS per well, and incubate for 2 minutes.
(2) Gently pipette to disrupt the matrix, collect the contents into a 15 ml centrifuge tube, adjust the volume to 10–14 ml with PBS, and incubate at 4°C for 20–30 minutes (group every 3–5 wells together). Centrifuge at 1000 rpm for 5 minutes, discard the supernatant, and retain the pellet.
(3) Resuspend the organoids in an appropriate amount of matrix, plate 25–30 µl per well in a 24-well cell culture plate, and incubate for 20–30 minutes to allow gelation. Add an appropriate amount of complete medium (expansion) for mouse cholangiocyte organoids for culture.
3. Cryopreservation of Organoids
(1) Aspirate the medium using a pipette, add 1–2 ml of 4°C PBS per well, and incubate for 2 minutes.
(2) Gently pipette to disrupt the matrix, collect the contents into a 15 ml centrifuge tube, adjust the volume to 10–14 ml with PBS, and incubate at 4°C for 20–30 minutes (group every 3–5 wells together). Centrifuge at 1000 rpm for 5 minutes, discard the supernatant, and retain the pellet.
(3) Add an appropriate amount of organoid cryopreservation medium, gently resuspend by pipetting, and aliquot. For a 24-well plate, cryopreserve the equivalent of 2–3 wells per cryovial, with 1 ml per vial.
(4) Label the vials appropriately, perform programmed freezing, and transfer to liquid nitrogen for long-term storage.
4. Thawing of Organoids
(1) Pre-aliquot 10 ml of DMEM/F12 basal medium into a 15 ml centrifuge tube.
(2) Retrieve the cryopreserved organoids from the liquid nitrogen tank and quickly thaw them in a 37°C water bath.
(3) During thawing, gently swirl the cryovial to ensure complete dissolution of the cryopreservation medium within 1–2 minutes.
(4) Quickly transfer the thawed organoids to the 15 ml centrifuge tube, gently pipette 6–8 times, centrifuge at 1000 rpm for 5 minutes, then discard the supernatant and collect the organoid pellet.
(5) Resuspend in matrix, plate 25–30 µl per well in a 24-well cell culture plate, and incubate for 20–30 minutes to allow gelation. Add an appropriate amount of complete medium (expansion) for mouse cholangiocyte organoids for culture.
Guidelines
Avoid vortex oscillation; store in separate containers to reduce the number of freeze-thaw cycles.
Picture
Picture
Bioactivity