| Usage |
1. Preparation:
1, Reagent Kit Preparation:
Keep the kit components on ice or 4℃, make sure it is fully dissolved before starting the operation.
2, Consumables and equipment required:
Fluorescence quantification PCRInstrument, vortex instrument, centrifuge
High-precision pipettes and disposable sterile, enzyme-free, low-adsorption filter tips (1-2.5 μL、0.5-10 μL、10-100 μL、20-200 μL、100-1000 μL) 5 mLSterile enzyme-free low-adsorption centrifuge tubes
Sterile enzyme-free eight-tube strip or 96hole qPCRplate
2. Operation process:
1Detailed operation steps:
(1) Extraction of test samples:
Recommended use“Host cell residues DNA(Magnetic beads method) Sample pretreatment kit (abs60544)”Extract samples DNAWhen adding the sample, add 4μLof Internal ControlWhen extracting samples, it is recommended to extract them synchronously DNAThe dilution served as a negative control.
(2) qPCRPreparation of reaction solution:
①According to the number of reference materials and samples to be tested (usually 2-3Calculate the number of wells required for the reaction:
Number of reaction wells=(1No-template control NTC+1Positive control PC+Sample to be tested) ×Number of multiple holes
②Calculate the required amount based on the number of reaction wells qPCR MIXTotal amount:
qPCR MIX =(Number of reaction wells+2or3)×12.5μL(2×qPCR Reaction MIX)+(Number of reaction wells +2or 3)×2.1μL(Mycoplasma Primer&Probe MIX)+(Number of reaction wells+2or3)×0.4μL(Internal Control)( 2or 3is the operating loss)
③Dissolve all reagents on ice, shake gently to mix, centrifuge briefly, and prepare as shown in the following table:
| Components |
Single reaction volume |
| 2×qPCR Reaction MIX |
12.5 μL |
| Mycoplasma Primer&Probe MIX |
2.1 μL |
| Internal Control |
0.4 μL |
| Total volume |
15 μL |
【Note】:If it is added during extractionIC, then prepareqPCR MIXWhen equal volume DNAdiluent instead IC.
④will be prepared qPCR MIX, gently shake to mix, centrifuge briefly, press15 μL/Hole divided into eight tubes or 96In the orifice plate.
⑤Will DNADiluent press 10 μL/The hole is added to the sub-packagingqPCR MIXEight-tube or96In the well plate, see the table below for specific sample additions.
Melted and unused DNAThe diluent can be 2-8If not used for a long time, please store at-20℃.
| Positive PC |
PCeach 10 μL+Required qPCR MIXquantity 15 μL |
| No template control NTC |
DNADilution 10 μL+Required qPCR MIXquantity 15 μL |
| Samples to be tested |
The samples to be tested 10 μL+Required qPCR MIXquantity 15 μL |
(3) qPCRAdd sample:
①Place the required reagents on ice, shake gently to mix, centrifuge briefly, and add the sample (total volume 25 μL):
②The experiment can use sterile enzyme-free eight-tube or 96When performing the reaction in the well plate, it is necessary to remove the bubbles in the reaction system and centrifuge to the bottom of the tube to prepare for the reaction.
(4) qPCRReaction program parameter settings:
The United States ABI 7500 Real-Time PCR System, software version 2.06For example.
A,exist Experimental PropertiesCreate a blank new program on the page as shown below:
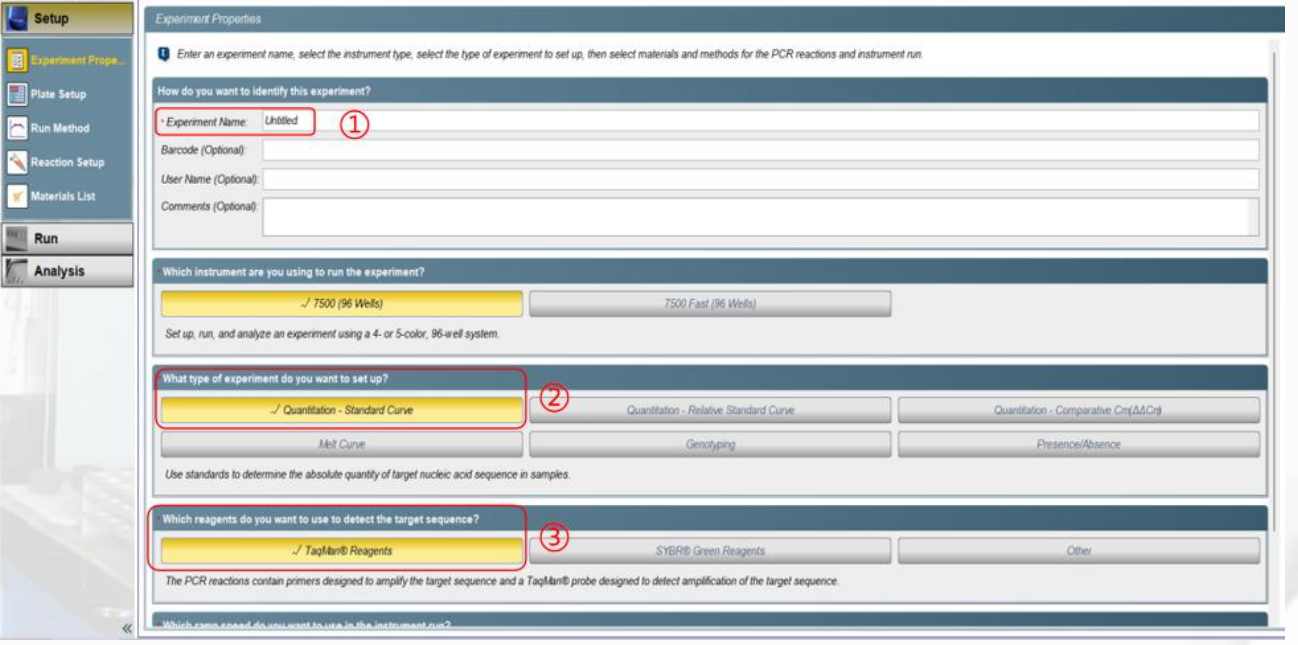
①Experiment name: Modify the experiment name.②Experiment type: Select a program (Quantitation-Standard Curve).③Reagents: Select the experimental method (TaqMan® Reagents).
B, create the product detection probe, selectselect ①interface, click ①interface, click ②Create a detection probe and an internal reference probe. TargetNamemiddleEdit the probe name and name it.Reporter③andQuencher④Select the desired fluorophore and quencherThis product detects fluorescent groups.FAM, the quenching group isNone, selection of internal reference fluorescent groupsCY5, the quenching group isNone, the reference fluorescence isROX.pointhit ②Create a detection probe and an internal reference probe. TargetNamemiddleEdit the probe name and name it.Reporter③andQuencher④Select the desired fluorophore and quencherThis product detects fluorescent groups.FAM, the quenching group isNone, selection of internal reference fluorescent groupsCY5, the quenching group isNone, the reference fluorescence isROX.pointhit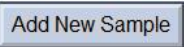 ⑤Create samples, in the corresponding Sample name⑥Name the sample in the column. ⑤Create samples, in the corresponding Sample name⑥Name the sample in the column.

C,existPlate Setup  ①Interface, place the positive control wells at Task②onecolumnset upPlacefor “S”③, the no-template control NTCKong ZaiTask②One column is set to“N”④, place the sample hole to be tested inTask②One column is set to “U”⑤, and in the corresponding Sample nameName the sample in a column (Figure LegendonlyforGinsengTest). ①Interface, place the positive control wells at Task②onecolumnset upPlacefor “S”③, the no-template control NTCKong ZaiTask②One column is set to“N”④, place the sample hole to be tested inTask②One column is set to “U”⑤, and in the corresponding Sample nameName the sample in a column (Figure LegendonlyforGinsengTest).
D, set up the reaction program:
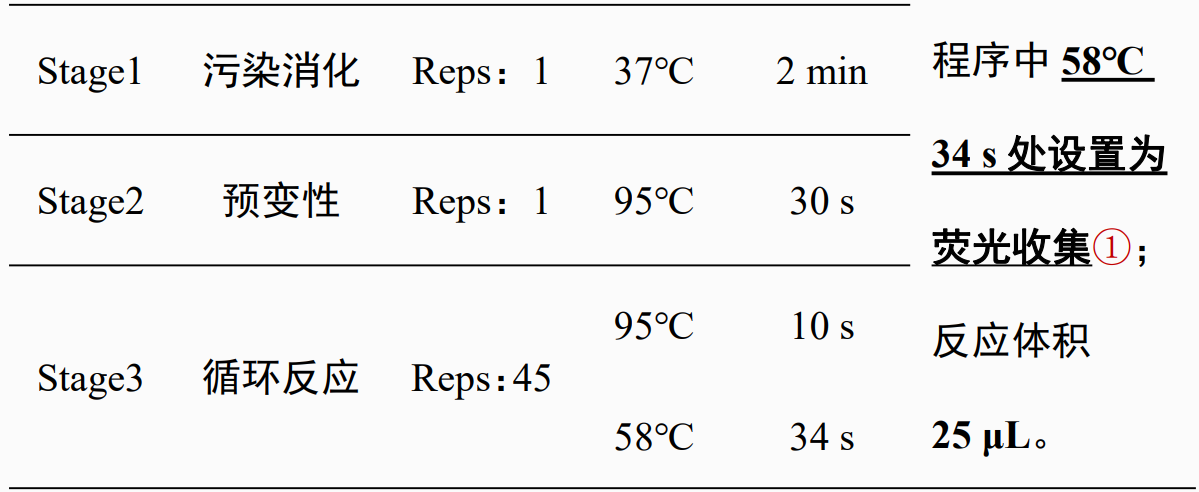
【Note】Some devices do not allow the fluorescence collection step to be set to 30sor shorter; use ABI7000、 ABI 7300and ABI 7500At least 31sFor other equipment, please consult the instrument manufacturer.
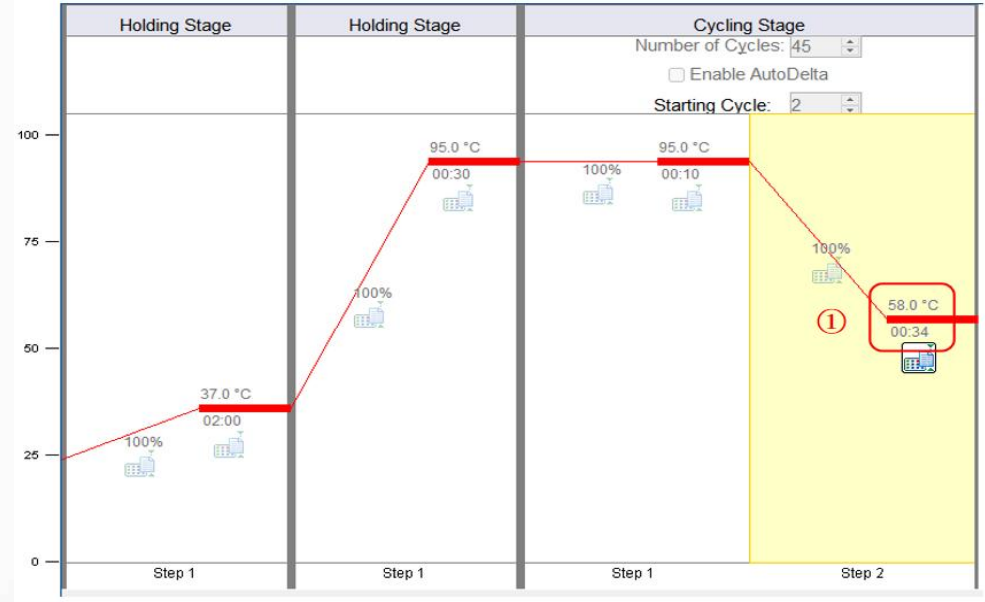
E, click RunInterface "Start Run"Button to proceedPCRDetermination.
3. Result Analysis
The United States ABI 7500 Real-Time PCR System, software version 2.06For example.
1,exist Analysisof Amplification Plot①In the panel, clickReanalyse②, initially check whether the shape of the amplification curve is normal. AnalysisofViewWell Table③In the panel, CtOne column can read the sample to be tested CtDetection value. (The illustration is for reference only)
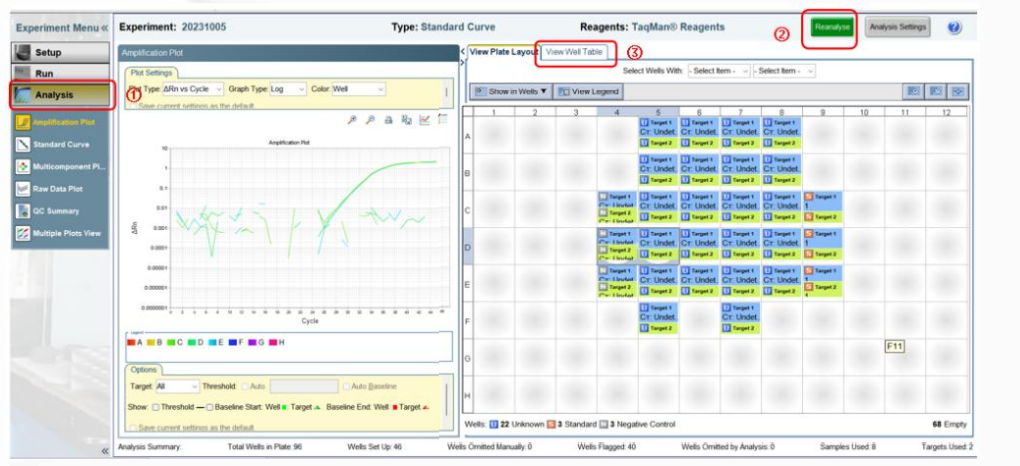
2The parameter settings for result analysis need to be based on the specific model and software version used, and can generally be automatically interpreted by the instrument.
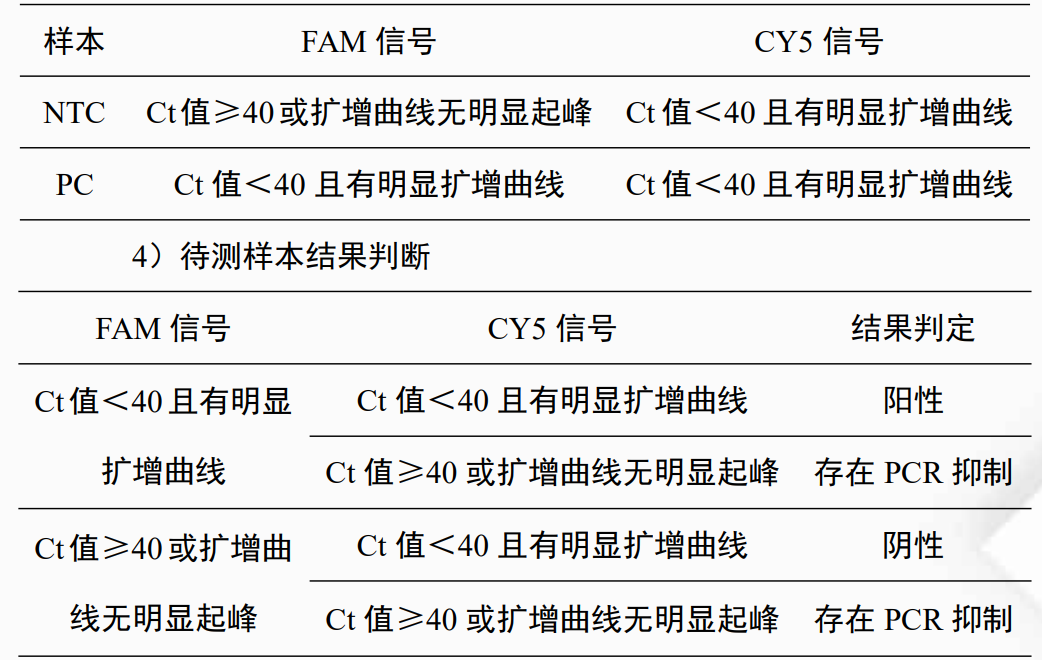
3、NTC,Positive PCThe results must meet
|
| Description |
Mycoplasmas are prokaryotes that commonly contaminate cells in the laboratory. The main sources of contamination include cells and virus strains, as well as animal-derived raw materials such as serum, chicken embryos, and primary cells, and the operators themselves. According to statistics, approximately 15%-35% of cells are contaminated with mycoplasmas. Mycoplasmas can alter a range of host cell characteristics, including structure and function, leading to inaccurate experimental results. They can also interfere with cell metabolism and growth, alter nucleic acid synthesis, affect cell antigenicity, cause chromosomal changes, interfere with viral replication, and mimic viral functions.
This test kit offers high specificity and sensitivity, and can be used to qualitatively detect mycoplasma contamination in master cell banks, working cell banks, viral seed batches, control cells, and cells used in clinical treatment. It can be used in conjunction with the Host Cell Residual DNA (Magnetic Bead Method) Sample Pretreatment Kit (abs60544). Detection Principle: According to <2.6.7> of the European Pharmacopoeia, nucleic acid amplification (NAT) can replace culture and indicator cell culture methods after appropriate validation. Fluorescent probe (TaqMan®) qPCR is characterized by high sensitivity and specificity, making TaqMan® the preferred method for mycoplasma detection. TaqMan® fluorescent probes are oligonucleotide probes with a fluorescent group (reporter) at the 5' end, such as FAM, CY5, or VIC, and a quencher at the 3' end, such as TAMRA or BHQ1. During PCR amplification, a specific fluorescent probe is added along with a pair of primers. When the probe is intact, the fluorescent signal emitted by the fluorescent group is absorbed by the quencher. During PCR amplification, the 5'-3' exonuclease activity of the Taq enzyme cleaves and degrades the probe, separating the fluorophore and quencher. This allows the fluorescence monitoring system (fluorescence quantitative PCR instrument) to detect a fluorescent signal. This means that each time a DNA strand is amplified, a fluorescent molecule is formed, ensuring that the accumulation of the fluorescent signal is completely synchronized with the formation of PCR products. This kit utilizes a multiplex PCR approach, using two different fluorescent probes to detect the target sequence and the internal reference, respectively.
Product components:
| Number |
Name |
Specifications |
Storage conditions |
| 1 |
Mycoplasma Primer&Probe MIX |
120 µL |
-20℃ and below light protection |
| 2 |
2×qPCR Reaction MIX |
700 & micro; height: 22px; text-align: center;">3 |
Internal Control |
220 µL |
-20℃ and below |
| 4 |
Positive Control |
500 & micro; center;">5 |
DNA Dilution 1.5 mL × 3 -20°C or below |
| Technical Index |
Limit of Detection: 100% detection rate for 10 standard strains of Mycoplasma species listed in various pharmacopoeias, including Mycoplasma oralis, Mycoplasma hyorhinis, and Mycoplasma pneumoniae. Limit of Blank: No detection was detected in 24 replicates of DNA diluted directly. Precision: Ten replicates of the same Mycoplasma-positive reference sample yielded positive results, with a coefficient of variation of Ct values of <5%. Specificity: No detection was detected when 300 pg/μL genomic DNA from common biological products (e.g., Human, Vero, CHO, HEK293, E. coli, Pichia pastoris) was used as a sample. No detection was detected when DNA from other engineered bacteria (e.g., Lactobacillus acidophilus and Lactobacillus fermentum) was used as a sample. No detection was detected when DNA from negative matrices such as PBS and 10% fetal bovine serum was used as a sample.
Durability:
Mycoplasma standard strains can be stably detected under different dilution matrices (such as DMEM medium, CHO serum-free medium, etc.), product freeze-thaw, product transportation, and multiple instrument model changes (such as ABI 7500, Bio-Rad CFX Opus96, Roche LightCycler 96, etc.). |
| General Notes |
(1) This kit has passed the stability verification (freeze-thaw and other factors) and does not require repackaging.
(2) The preparation and loading environment of negative samples (2×qPCR Reaction MIX, Primer & Probe MIX and DNA diluent, etc.) and positive samples (PC and test samples, etc.) must be separated into different areas and cannot be operated in the same area. The preparation personnel must dress neatly, wear masks, gloves and clean clothes.
(3) Pay attention to changing the pipette tips in time between different loading steps to avoid cross contamination, avoid opening the lid for a long time, and avoid liquid sticking to the wall when using a pipette.
(4) The kit must be used within the validity period. It is not recommended to mix related reagents from different batches.
(5) It is recommended that all components in the kit be thawed in a low temperature environment before use, and it must be ensured that they are fully dissolved. Each component must be fully mixed before use to ensure the homogeneity of the reagents. The mixed reagents must be briefly centrifuged so that all the liquid on the tube wall and lid is concentrated at the bottom of the tube. If precipitates are found in the DNA diluent, it is recommended to incubate at 37°C to completely dissolve the DNA diluent.
(6) Only by strictly following the operating instructions and using all the reagents provided with this kit can the best detection results be guaranteed.
(7) The final test results are closely related to the effectiveness of the reagents, the operator's operating methods, and the test environment. |
| Storage Temp. |
Store at -20℃, valid for 12 months. |
|









