| Usage |
1. Experimental instruments and materials
Auxiliary reagent
| |
Product name |
Item number |
Specifications |
Storage conditions and cycles |
| 1 |
PBS |
abs962 |
500mL |
Stored at 2 ~ 8 ℃ or room temperature, the validity period is 12 months. |
| 2 |
Advance human pluripotent stem cell culture medium |
abs9404 |
100mL |
Store at-20 ℃, shelf life is 2 years. |
| 3 |
Human pluripotent stem cell digestive juice |
abs9409 |
100mL |
Stored at-20 ℃, shelf life is 2 years. |
Experimental consumables
Cell culture plate (standard clear 24-well plate)
| |
Product name |
Item number |
Specifications |
Storage conditions and cycles |
| 1 |
Cell culture plate (standard clear 6-well plate) |
abs7033 |
1 box |
Room temperature, shelf life 3 years. |
| 2 |
Cell Culture Plate (Standard Clear 12 Well Plate) |
abs7034 |
1 box |
Room temperature, shelf life 3 years. |
| 3 |
Cell culture plate (standard clear 24-well plate) |
abs7035 |
1 box |
Room temperature, shelf life 3 years. |
| 4 |
1.5 mL Centrifuge tube |
abs7119 |
1 box |
Room temperature, shelf life 3 years. |
| 5 |
15 mL Centrifuge tube |
abs7120 |
1 box |
Room temperature, shelf life 3 years. |
| 6 |
50 mL Centrifuge tube |
abs7121 |
1 box |
Room temperature, shelf life 3 years. |
| 8 |
10mL disposable pipette |
abs7053 |
1 box |
Room temperature, shelf life 3 years. |
| 9 |
50mL disposable pipette |
abs7055 |
1 box |
Room temperature, shelf life 3 years. |
| 10 |
10uL tips (boxed, sterile and enzyme-free) |
abs7072 |
1 box |
Room temperature, shelf life 3 years. |
| 11 |
200uL tips (boxed, sterile and enzyme-free) |
abs7075 |
1 box |
Room temperature, shelf life 3 years. |
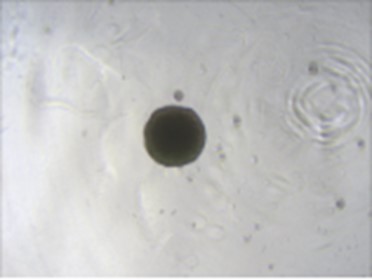
2. Induction flow chart
 |
 |
| Phase 1 |
Stage 2 |
 |
 |
| Three stages |
Four stages |
3. Kit usage process
① EB formation (2 days)
1. hPSC was cultured in one well of a six-well plate coated with Matrigel and proliferated with Advance human pluripotent stem cell culture medium to 70-80% confluence.
2. Aspirate the Advance human pluripotent stem cell culture medium.
3. Add 1mL PBS (no calcium and magnesium ions) to the wells, wash and aspirate.
4. Add 1mL of human pluripotent stem cell digestion juice to the well, and digest at 37 ℃ for 3min.
520 mL of EB forming medium and 40 μL of EB forming supplement were uniformly mixed to obtain EB forming complete medium.(Note: The culture medium used should be placed on the workbench for 30min in advance to restore room temperature, and the same is true for the following operations.)
6. After digestion, the clone can be seen white and bright under the microscope.
7. Aspirate the digestion juice of human pluripotent stem cells, and add 1mL of EB to each well to form a complete medium to terminate digestion.
8. Gently blow the cells in the well 2-3 times, and transfer 500uL of the cell suspension to a 15mL centrifuge tube with 9mLEB forming medium.
9. Centrifuge at 100g for 5min, and aspirate and discard the supernatant.
10. Flick the cell pellet, add one ml of EB to form complete medium to resuspend the cells, add 9mLEB to the cell suspension to form complete medium, and make a cell suspension again.
11. Transfer the cell suspension to the sample loading tank, use a multi-channel pipette to plant it into an ultra-low adsorption 96 plate, 100μL per well, inoculate 96 wells, add 100μL PBS per well of an ordinary 96-well plate, and 96 wells are used as trim.
12. Centrifuge in a low-speed horizontal centrifuge at 850rpm/120g for 3min, put the plate in 37 °C, 5% CO2The incubator is denoted as D-2.
13. The formation of EB with uniform size can be seen on day 2 (D-1). Each well was supplemented with 100 μL of EB-forming medium (without supplement) and placed at 37 °C, 5% CO2The incubator.
(Note: After replenishing the EB-forming medium, the multi-channel pipette tip can be placed under the liquid surface and gently blown to mix the medium without affecting the EB.)
② EB differentiation stage (12 days)
14. D0, thaw supplement A, 6 mL of pre-cooled basal medium 1 and supplement A and 200 μL of Matrigel are mixed evenly. After returning to room temperature, the medium is changed at 100 μL per well to induce EB differentiation, and the EB is suspended by gently pipetting with a multi-channel pipette. At 37 °C, 5% CO2Incubate in an incubator for 4 days.
15. D4, thaw supplement B, 2.5 mL of basal medium 1 + supplement B and mix evenly, add it to a 96-well plate at 25 μL per well, and gently pipette 8-10 times to mix evenly, so that the final volume of medium per well is 125 μL, 37 ℃, 5% CO2 Incubate for 4 days.
16. D8, thaw supplement C, 2.5 mL of basal medium 1 + supplement C and mix evenly, add it to a 96-well plate at 25 μL per well, and gently pipette 8-10 times to mix evenly, so that the final volume of each well medium is 150μL, and the 96-well plate is returned to the incubator, 37 ℃, 5% CO2Incubate for 4 days.
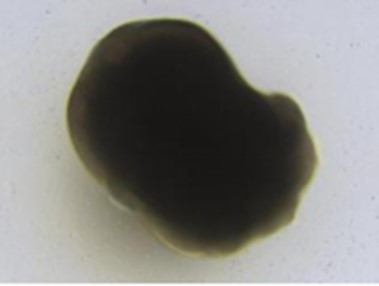
③ Ear bubble formation stage (6 days)
17. D12, after EB induction, prepare basal medium 2 (refrigerate on ice for at least 30 minutes in advance); Alternatively, basal medium 2 was stored at 4 ° C. overnight on D11. Matrigel was thawed with supplement D at 4 °C in advance, and the cold basal medium 2 was evenly mixed with supplement D. 200 uL of Matrigel was added to 20 mL of cold mixed medium, repeatedly pipetted and dissolved 20 times, and rewarmed to room temperature. The aggregates in the 96-well plate were transferred to two 100 mm dishes with 48 aggregates in each dish. The aggregates were washed twice with 1-2 mL of PBS and once again with 1 mL of mixed medium without Matrigel, then 10 mL of mixed medium containing Matrigel were added respectively, and the dishes were placed in an incubator at 37 °C, 5% CO2Incubation for 3 days; On D15, the culture medium in the petri dish was aspirated, the mixed medium without Matrigel was readded, and the culture was continued for 3 days to induce
Ear bubble formation;
(Note: Generally, 10mL of culture medium is needed for solution change of 10mm dish)
④ Maturation stage of inner ear organoids (42 days)
18. D18-60, inner ear organoids gradually mature, change the medium to mature medium, start long-term culture, change the fluid every 5 days, and obtain mature inner ear organoids with sensory hair cells in about 60 days.
|






