Product Details
Product Details
Product Specification
| Usage |
Self-prepared test equipment required for the experiment: 1. Microplate reader (450nm) 2. High-precision sampler and gun tip: 0.5-10uL, 5-50uL, 20-200uL, 200-1000uL 3. 37 ℃ incubator 4. Distilled water or deionized water Sample handling and requirements: Serum: Place the whole blood sample collected in the serum separation tube at room temperature for 2 hours or 4 ℃ overnight, then centrifuge at 1000 × g for 20 minutes, take the supernatant, or store the supernatant at-20 ℃ or-80 ℃, but avoid repeated freezing and thawing. Plasma: Collect specimens with EDTA or heparin as anticoagulants, and centrifuge the specimens at 2-8 ℃ 1000 × g for 15 minutes within 30 minutes after collection. Take the supernatant for detection, or place the supernatant at-20 ℃ or-80 ℃ for storage, but avoid repeated freezing and thawing. Tissue homogenate: Wash the tissue with pre-cooled PBS (0.01 M, pH = 7.4) to remove residual blood (lysed red blood cells in the homogenate will affect the measurement results), and break the tissue into pieces after weighing. Combine the crushed tissue with the corresponding volume of PBS (generally according to the weight-to-volume ratio of 1: 9, for example, 1g of tissue sample corresponds to 9mL of PBS. The specific volume can be appropriately adjusted according to the experimental needs and recorded. It is recommended to add protease inhibitor to PBS) Add to a glass homogenizer and grind thoroughly on ice. For further lysis of tissue cells, the homogenate can be sonicated, or freeze-thawed repeatedly. Finally, the homogenate was centrifuged at 5000 × g for 5-10 minutes, and the supernatant was taken for detection. Cell lysate: Adherent cells were gently washed with pre-cooled PBS, then digested with trypsin, centrifuged at 1000 × g for 5 minutes, and the cells were collected; The suspended cells can be collected directly by centrifugation. The collected cells were washed 3 times with pre-cooled PBS, and 150-200uL PBS was added to every 1 × 10 ^ 6 cells to resuspend (it is recommended to add protease inhibitors to PBS; if the content is very low, the PBS volume can be appropriately reduced) and the cells were disrupted by repeated freezing and thawing or ultrasound. The extract was centrifuged at 2-8 °C at 1500 × g for 10 minutes, and the supernatant was taken for detection. Cell culture supernatant: Please centrifuge at 1000 × g for 20 minutes, take the supernatant for detection, or store the supernatant at-20 ℃ or-80 ℃, but avoid repeated freezing and thawing. Liquid Other biological liquids: centrifuge at 1000xg for 20 minutes, and take the supernatant for detection. Preparations before testing: 1. Please take out the kit from the refrigerator 10 minutes in advance and balance it to room temperature. 2. Preparation of standard gradient working solution: Add 1mL of universal diluent to the freeze-dried standard, let it stand for 15 minutes until it is completely dissolved, and then gently mix (the concentration is 500pg/mL), and then dilute according to the following concentrations: 500pg/mL, 250pg/mL, 125pg/mL, 62.5 pg/mL, 31.25 pg/mL, 15.625 pg/mL, 7.8125 pg/mL, 0pg/mL. Double dilution method: Take 7 EP tubes, add 500uL of universal diluent to each tube, draw 500uL of 500pg/mL standard working solution into the first EP tube and mix well to prepare 250pg/mL standard working solution. According to this step, draw and mix well in sequence. The last tube is directly used as a blank hole, so there is no need to suck liquid from the penultimate tube, as shown in the figure below. 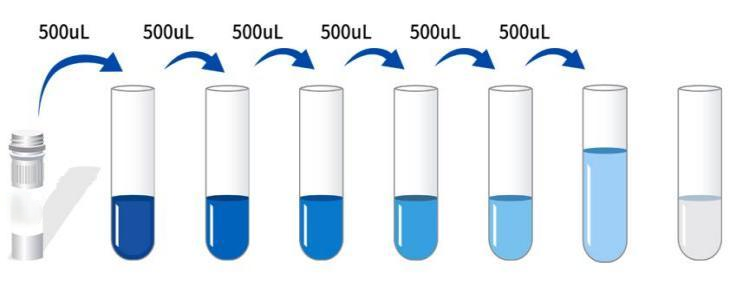 3. Preparation of biotinylated antibody detection working solution: 15 minutes before use, centrifuge the concentrated biotinylated antibody at 1000 × g for 1 minute, and dilute 100 × concentrated biotinylated antibody to 1 × working concentration with universal diluent (example: 10uL concentrated solution + 990uL universal diluent), now prepared for use. 4. Preparation of enzyme conjugate working solution: Centrifuge 100 × concentrated enzyme conjugate at 1000 × g for 1 minute 15 minutes before use, and dilute 100 × concentrated HRP enzyme conjugate to 1 × working concentration with universal diluent (example: 10uL concentrated solution + 990uL universal diluent), now prepared for use. 5. Preparation of 1 × washing liquid: Take 10mL of 20 × washing liquid into 190mL distilled water (the concentrated washing liquid taken out of the refrigerator may have crystals, which is a normal phenomenon. It can be placed at room temperature and then prepared after the crystals are completely dissolved). Operation steps: 1. Take out the required slats from the aluminum foil bag after equilibration at room temperature for 10 minutes, and seal the remaining slats with a ziplock bag and put them back to 4 °C. 2. Add samples: Add samples or standards of different concentrations to the corresponding wells at 100uL per well, and add 100uL of universal diluent to the blank wells. Incubate at 37 °C for 60 minutes after covering the plate sealing film. (Recommendation: Dilute the sample to be tested with a universal diluent at least 1 times and then add it to the enzyme labeled plate for testing. This reduces the influence of matrix effect on the test results. Finally, the sample concentration needs to be multiplied by the corresponding dilution factor when calculating. It is recommended to set up double wells for all samples and standards to be tested during testing). 3. Add biotinylated antibody: take out the enzyme labeled plate, discard the liquid, and do not wash it. 100 uL of biotinylated antibody working solution was directly added to each well, and the plate sealing membrane was covered and incubated at 37 °C for 60 minutes. 4. Plate washing: Discard the liquid, add 300uL 1x washing liquid to each hole, let it stand for 1 minute, throw away the washing liquid, pat dry on absorbent paper, and repeat washing the plate 3 times (you can also use a plate washing machine to wash the plate). 5. Add enzyme conjugate working solution: Add 100uL of enzyme conjugate working solution to each well, cover the sealing membrane and incubate at 37 °C for 30 minutes. 6. Wash the plate: Discard the liquid and wash the plate 5 times according to the washing method in step 4. 7. Add substrate: Add 90uL of substrate (TMB) to each well, cover with a sealing film, and incubate at 37 °C in the dark for 15 minutes. 8. Add stop solution: Take out the enzyme plate, directly add 50uL of stop solution to each well, and immediately measure the OD value of each well at a wavelength of 450nm. Calculation of experimental results: Result judgment: 1. Calculate the average OD value of the standard product and the sample double well and subtract the OD value of the blank well as the correction value. Taking concentration as abscissa and OD value as ordinate, the standard curve of four-parameter logic function is drawn on double logarithmic coordinate paper. 2. If the OD value of the sample is higher than the upper limit of the standard curve, it should be properly diluted and retested and multiplied by the corresponding dilution factor when calculating the sample concentration. 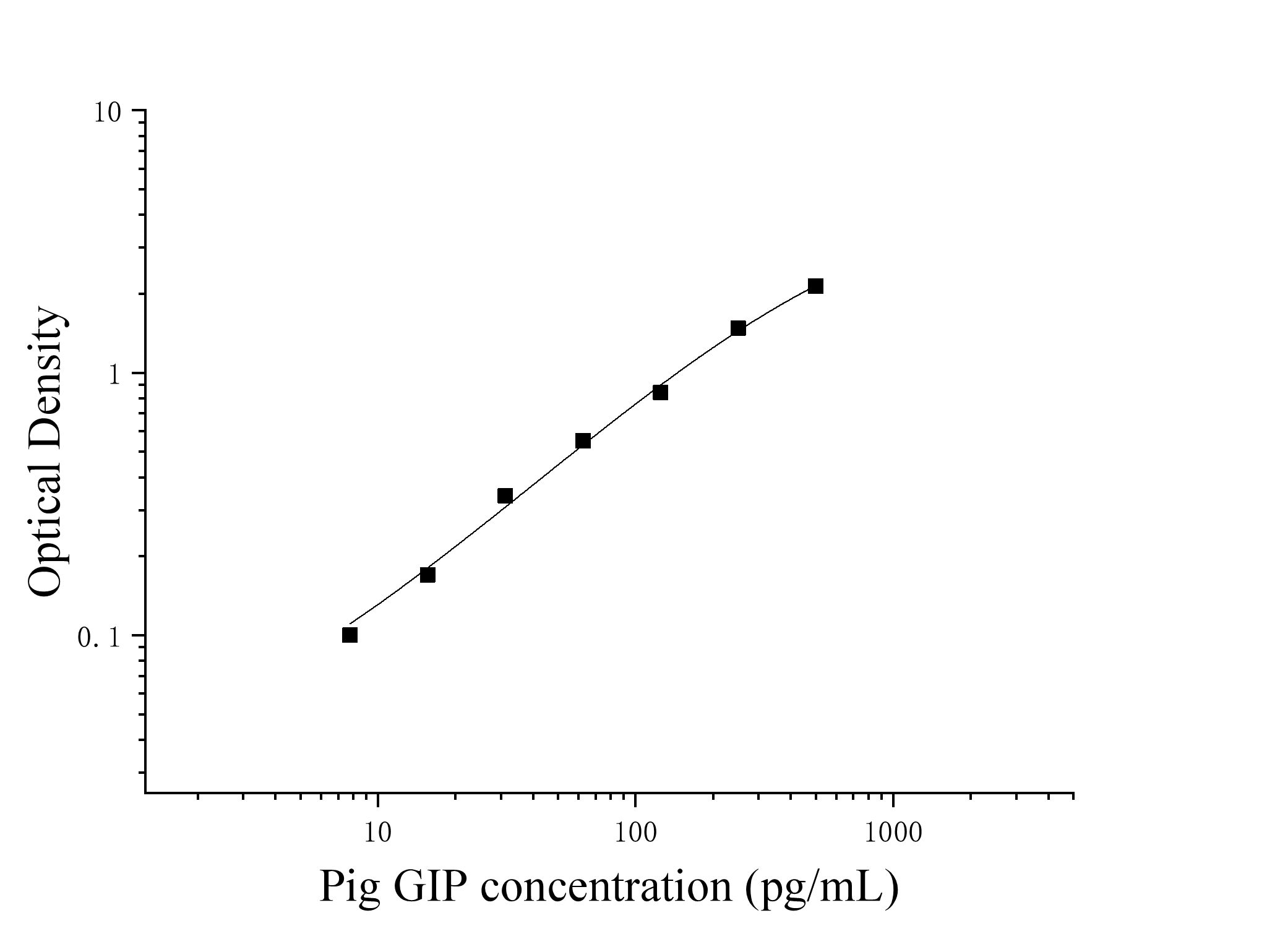
|
|||||||||||||||||||||||||||||||||
| Theory | This kit uses double antibody sandwich enzyme-linked immunosorbent assay (ELISA). To the microwells pre-coated with Gastric Inhibitory Polypeptide (GIP) capture antibody, sample, standard product, biotin-labeled detection antibody, and HRP enzyme conjugate were added in sequence, incubated and washed in the middle, and colored with substrate TMB. TMB is converted to blue under the catalysis of peroxidase (HRP) and to the final yellow under the action of acid. There was a positive correlation between the depth of color and the Gastric Inhibitory Polypeptide (GIP) in the sample. The absorbance (0D value) was measured with a microplate reader at a wavelength of 450nm, and the sample concentration was calculated. | |||||||||||||||||||||||||||||||||
| Source | Porcine | |||||||||||||||||||||||||||||||||
| Synonym | Pig Gastric Inhibitory Polypeptide ELISA Kit | |||||||||||||||||||||||||||||||||
| Detection Type | Double antibody sandwich method | |||||||||||||||||||||||||||||||||
| Composition |
|
|||||||||||||||||||||||||||||||||
| Background | 0 | |||||||||||||||||||||||||||||||||
| General Notes | 1. Carry out incubation in strict accordance with the specified time and temperature to ensure accurate results. All reagents must reach room temperature 20-25 °C prior to use. Store reagents in refrigeration immediately after use. 2. Incorrect plate washing may lead to inaccurate results. Make sure to drain the liquid from the wells as much as possible before adding the substrate. Do not allow the wells to dry out during incubation. 3. Eliminate the residual liquid and fingerprints at the bottom of the plate, otherwise it will affect the OD value. 4. The substrate color development solution should be colorless or very light in color, and the substrate solution that has turned blue cannot be used. 5. Avoid cross-contamination of reagents and specimens to avoid wrong results. 6. Avoid direct exposure to strong light during storage and incubation. 7. Any reaction reagent cannot come into contact with the bleaching solvent or the strong gas emitted by the bleaching solvent. Any bleaching component will destroy the biological activity of the reaction reagents in the kit. 8. Expired products cannot be used, and components with different item numbers and batch numbers cannot be mixed. 9. Recombinant proteins from sources other than the kit may not match the antibodies in this kit and are not recognized. 10. If the disease may be spread, all samples should be managed well, and the samples and testing devices should be handled according to the prescribed procedures. |
|||||||||||||||||||||||||||||||||
| Storage Temp. | Unopened kit, stored at 4 °C, shelf life 6 months. | |||||||||||||||||||||||||||||||||
| Test Range | 7.8-500pg/mL | |||||||||||||||||||||||||||||||||
| Applications | Serum, plasma, tissue homogenates, cell lysates, cell culture supernatants and other biological fluids |