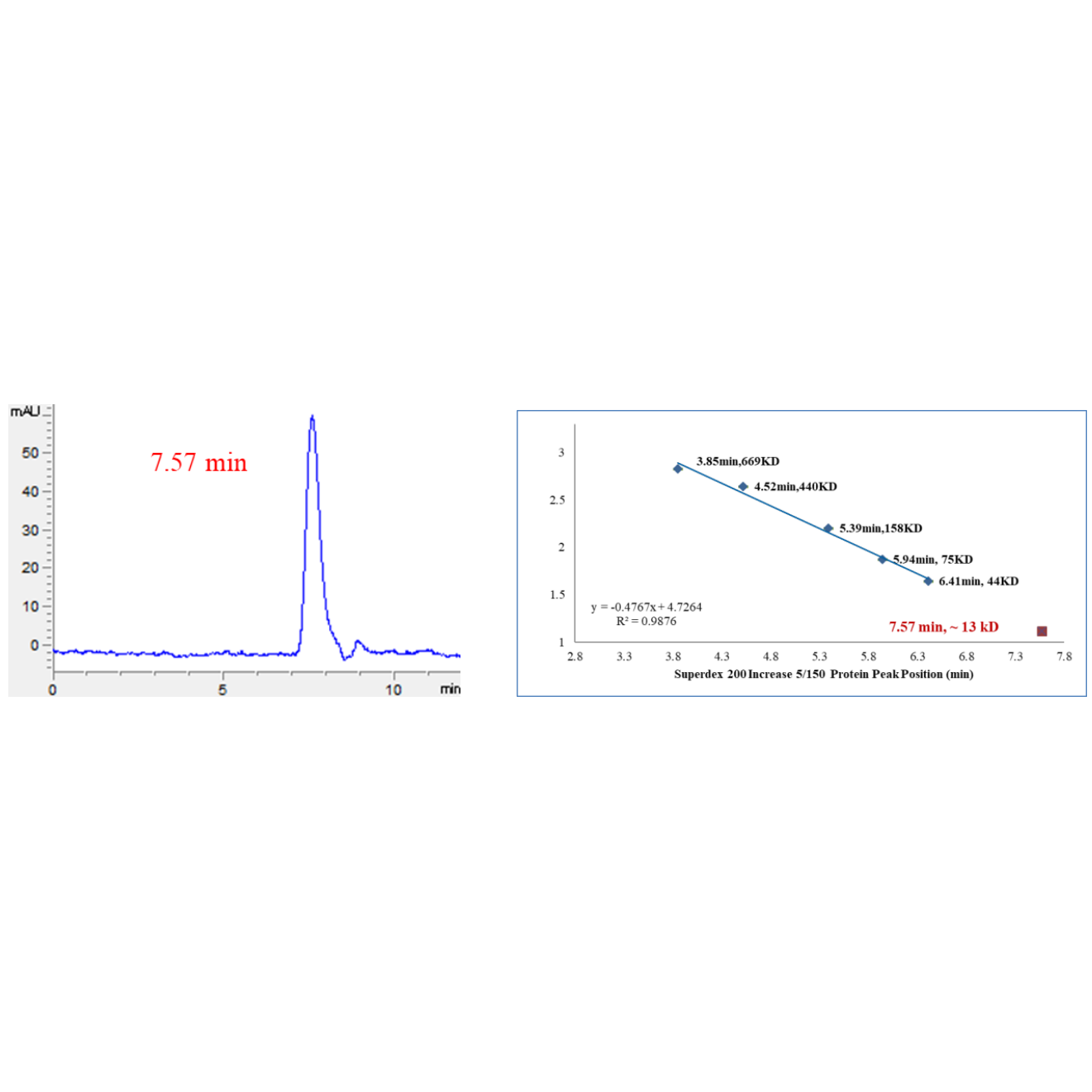
Lane M: Marker
Lane 1: 2 μg, reduced
Lane 2: 2 μg, non-reduced
Product Details
Product Details
Product Specification
| Species | Human |
| Synonyms | E3 ubiquitin-protein ligase Mdm2,Double minute 2 protein,Hdm2,Oncoprotein Mdm2,RING-type E3 ubiquitin transferase Mdm2,p53-binding protein Mdm2 |
| Accession | Q00987 |
| Amino Acid Sequence |
S17-N111 SQIPASEQETLVRPKPLLLKLLKSVGAQKDTYTMKEVLFYLGQYIMTKRLYDEKQQHIVYCSNDLLGDLFGVPSFSVKEHRKIYTMIYRNLVVVN |
| Expression System | E.coli |
| Purity | >90% by SDS-PAGE |
| Conjugation | Unconjugated |
| Tag | His Tag |
| Physical Appearance | Liquid |
| Storage Buffer | 50mM Tris-HCl (pH 7.5), 200mM NaCl, 20% glycerol |
| Stability & Storage | Samples are stable for up to twelve months from date of receipt at -20℃ to -80℃ |
Background
E3 ubiquitin-protein ligase that mediates ubiquitination of p53/TP53, leading to its degradation by the proteasome. Inhibits p53/TP53- and p73/TP73-mediated cell cycle arrest and apoptosis by binding its transcriptional activation domain. Also acts as a ubiquitin ligase E3 toward itself and ARRB1. Permits the nuclear export of p53/TP53. Promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma RB1 protein. Inhibits DAXX-mediated apoptosis by inducing its ubiquitination and degradation. Component of the TRIM28/KAP1-MDM2-p53/TP53 complex involved in stabilizing p53/TP53. Also component of the TRIM28/KAP1-ERBB4-MDM2 complex which links growth factor and DNA damage response pathways. Mediates ubiquitination and subsequent proteasome degradation of DYRK2 in nucleus. Ubiquitinates IGF1R and SNAI1 and promotes them to proteasomal degradation (PubMed:12821780, PubMed:15053880, PubMed:15195100, PubMed:15632057, PubMed:16337594, PubMed:17290220, PubMed:19098711, PubMed:19219073, PubMed:19837670, PubMed:19965871, PubMed:20173098, PubMed:20385133, PubMed:20858735, PubMed:22128911). Ubiquitinates DCX, leading to DCX degradation and reduction of the dendritic spine density of olfactory bulb granule cells (By similarity). Ubiquitinates DLG4, leading to proteasomal degradation of DLG4 which is required for AMPA receptor endocytosis (By similarity). Negatively regulates NDUFS1, leading to decreased mitochondrial respiration, marked oxidative stress, and commitment to the mitochondrial pathway of apoptosis (PubMed:30879903). Binds NDUFS1 leading to its cytosolic retention rather than mitochondrial localization resulting in decreased supercomplex assembly (interactions between complex I and complex III), decreased complex I activity, ROS production, and apoptosis (PubMed:30879903).
Picture
Picture
SDS-PAGE
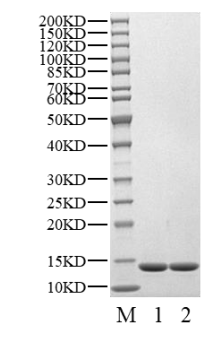
SEC-HPLC