Product Details
Product Details
Product Specification
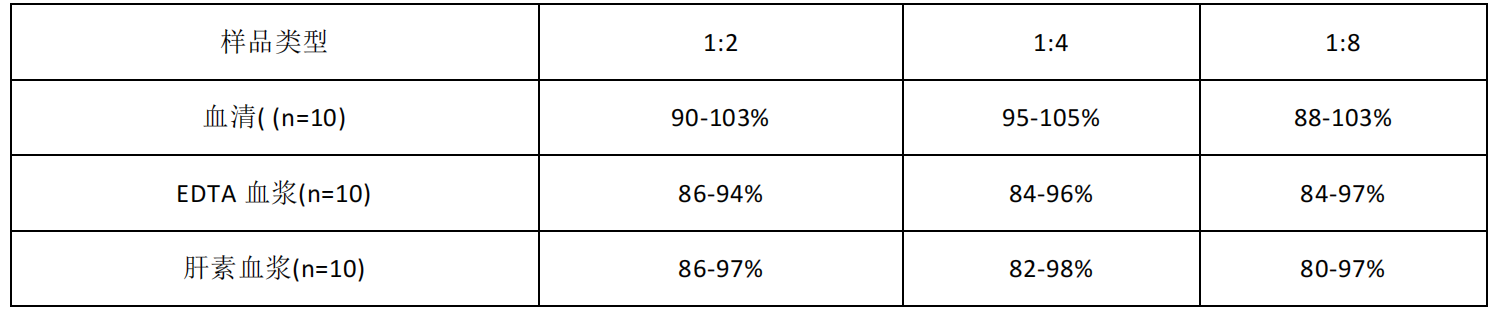
| Usage | Required Equipment and Reagents: 1. Microplate reader (450nm wavelength filter) 2. 37°C incubator (CO2 incubator for cells is not recommended) 3. Automatic plate washer or multichannel pipette/5ml dropper (for manual plate washing) 4. Precision single-channel (0.5-10 μL, 5-50 μL, 20-200 μL, 200-1000 μL) and multichannel pipettes (pipettes must be calibrated before use). 5. Sterile EP tubes and disposable pipette tips 6. Blotting paper and sample reservoir 7. Deionized or distilled water Sample Collection and Storage: 1SerumWhole blood samples should be incubated at room temperature for 2 hours or at 2-8°C overnight. Centrifuge at 1000×g for 20 minutes and remove the supernatant. The sample can be tested immediately or aliquoted for single use and frozen at -20°C or -80°C.2PlasmaEDTA-Na2/K2 is recommended as the anticoagulant. Within 30 minutes of sample collection, centrifuge at 1000×g for 15 minutes at 2-8°C and remove the supernatant. It can be tested immediately or aliquoted and frozen at -20°C or -80°C according to the amount used for one time. For the use and selection of other anticoagulants, please refer to the sample preparation guide. 3. Tissue samples Tissue samples are generally made into tissue homogenates. The processing method is as follows: (1) Place the target tissue on ice, wash it with pre-cooled PBS buffer (0.01M, pH=7.4) to remove residual blood, weigh it and set aside. (2) Grind the tissue homogenate with lysis buffer on ice. The volume of lysis buffer added depends on the weight of the tissue. Generally, 9ml of lysis buffer is used for every 1g of tissue fragments. It is also recommended to add protease inhibitors to the lysis buffer, such as 1mM PMSF. (3) It can be further processed by ultrasonic disruption or repeated freeze-thaw (during ultrasonic disruption, an ice bath is required to cool down; repeated freeze-thaw method can be repeated twice). (4) Centrifuge the prepared homogenate at 5000×g for 5 minutes and keep the supernatant for testing. Alternatively, aliquot the sample into a single-use amount and freeze at -20°C or -80°C. (5) Depending on the experimental needs, the total protein concentration of the tissue homogenate sample can be measured first to facilitate data analysis. The BCA method is recommended. Generally, the total protein concentration is adjusted to 1-3 mg/ml for ELISA testing. Some tissue samples, such as liver, kidney, and pancreas, contain high concentrations of endogenous peroxidase. When the sample concentration is high, they will react with the chromogenic substrate and produce false positives. You can try to use 1% H2O2 to inactivate for 15 minutes before testing. Note: The lysis buffer is usually PBS buffer, or a medium-strength RIPA lysis buffer is used. When using RIPA lysis buffer, adjust the pH to 7.3. Avoid using components containing NP-40, Triton X-100, and DTT, as these will severely inhibit the kit's performance. We recommend 50mM Tris + 0.9% NaCl + 0.1% SDS, pH 7.3. You can prepare your own or contact us to purchase. 4. Cell Culture Supernatant: Collect the supernatant and centrifuge at 2500 rpm for 5 minutes at 2-8°C. Collect the clarified cell culture supernatant. Use immediately for analysis or aliquot and freeze at -80°C for later use. 5. Cell Lysis Solution (1) Collection and Lysis of Suspended Cells: Centrifuge at 2500 rpm for 5 min at 2-8°C to collect cells. Then add pre-cooled PBS and gently mix and wash. Centrifuge at 2500 rpm for 5 min at 2-8°C to collect cells. Add 0.5-1 ml of cell lysis solution and an appropriate amount of protease inhibitor (such as PMSF, working concentration 1 mmol/L), place on ice, and lyse for 30 min-1 h, or use ultrasonic disruption. (2) Collection and Lysis of Adherent Cells: Aspirate the supernatant and add pre-cooled PBS to wash three times. Add 0.5-1 ml of cell lysis solution and an appropriate amount of protease inhibitor (such as PMSF, working concentration 1 mmol/L), and gently scrape the adherent cells with a cell scraper. Transfer the cell suspension to a centrifuge tube, place on ice, and lyse for 30 min-1 h, or use ultrasonic disruption. (3) During the cell lysis process, you can use the tip of the pipette to blow or shake the centrifuge tube intermittently to fully lyse the protein. If a sticky substance appears, it is DNA, which can be broken by ultrasound. (Or use an ultrasonic 3-5mm probe, power 150-300W, sonicate the sample on ice, work for 1-2 seconds, stop for 30 seconds, and repeat 3-5 cycles.) (4) After lysis or ultrasonic disruption is completed, centrifuge at 2-8°C, 10000rpm for 10 minutes, transfer the supernatant into an EP tube, and use it immediately for detection, or divide it into aliquots according to the amount used once and freeze it at -80°C for later use. Note: The precautions are the same as those for tissue samples. 6Other biological samples Centrifuge the sample at 2-8°C, 1000×g for 20 minutes. Collect the supernatant and use it immediately for testing, or aliquot into single-use amounts and freeze at -80°C until further use. Additional Sample Precautions: 1. Blood collection tubes should be disposable, endotoxin-free tubes. Avoid using hemolyzed or hyperlipidemic samples. 2. Optimal Sample Storage Conditions: Samples should be stored at 2-8°C for less than 5 days, at -20°C for no more than 6 months, and at -80°C for no more than 2 years. After these storage periods, they should be stored in liquid nitrogen. When thawing frozen specimens, to minimize damage to the sample by ice crystals (0°C), rapidly thaw in a 15-25°C water bath. After thawing, centrifuge to remove the precipitate, and mix thoroughly before use for testing. 3. The detection range of the kit does not correspond to the concentration range of the analyte in the sample. If the analyte concentration in the sample is too high or too low, dilute or concentrate the sample appropriately. 4. If the sample being tested is unique and no reference data is available, it is recommended to perform a preliminary experiment to verify its validity. 5. The recombinant protein may not be compatible with the capture or detection antibodies in the kit, resulting in undetectable results. Recommended Sample Dilution Scheme: Matrix components in serum/plasma can affect test results. Dilute the sample at least 2-fold (1/2) with Sample Diluent! If your model group samples require a different dilution ratio, please refer to the following general dilution scheme (this scheme is for non-replicate wells. If replicate wells are required, multiply the sample and diluent volumes by the number of replicate wells): 2-fold (1/2) dilution: One-step dilution. Add 60µl of sample to 60µl of Sample Diluent and mix gently. 5-fold (1/5) dilution: One-step dilution. Add 24µl of sample to 96µl of Sample Diluent and mix gently. 10-fold (1/10) dilution: One-step dilution. Add 12µl of sample to 108µl of sample diluent and mix gently. 20-fold dilution (1/20): One-step dilution. Add 6µl of sample to 114µl of sample diluent and mix gently. 50-fold dilution (1/50): One-step dilution. Add 3µl of sample and 47µl of saline (i.e., 0.9% sodium chloride) to 100µl of sample diluent and mix gently. 100-fold dilution (1/100): One-step dilution. Add 3µl of sample and 177µl of saline to 120µl of sample diluent and mix gently. 1000-fold dilution (1/1000): Two-step dilution: First dilute 50-fold (using saline throughout this step), then dilute 20-fold. Mix gently. 10,000-fold (1/10,000) dilution: Perform a two-step dilution: dilute 100-fold first (use normal saline for all dilutions in this step), then dilute 100-fold. Mix gently. 100,000-fold (1/100,000) dilution: Perform a three-step dilution: dilute 50-fold first, then 20-fold (use normal saline for all dilutions in the first two steps), and finally dilute 100-fold. Mix gently. Note: For each dilution step, dispense at least 3 μl of solution, and the dilution factor should not exceed 100-fold. Mix thoroughly at each dilution step to avoid foaming. Pre-Assay Reagent Preparation: Remove the test kit from the refrigerator 20 minutes in advance and equilibrate to room temperature (18-25°C). If the kit is to be used multiple times, only remove the ELISA strips and standards needed for the current experiment. The remaining ELISA strips and standards should be stored according to the specified conditions. 1. Wash Solution: Dilute 30ml of concentrated wash solution (15ml for 48T) to 750ml (375ml for 48T) with deionized or distilled water (recommended ultrapure water with a resistivity of 18MΩ). Mix thoroughly. Alternatively, dilute an appropriate amount of concentrated wash solution to 25 times the volume as needed for the experiment, mix thoroughly, and return the unused solution to 2-8°C. If crystals form in the concentrated wash solution, heat it in a 40°C water bath (do not exceed 50°C) until the crystals are completely dissolved. Mix thoroughly before use. The prepared washing solution should be used up on the same day. If not used up, it can be stored at 2-8°C for no more than 48 hours. 2Standards: (1) Centrifuge the freeze-dried standard tube at 10,000×g for 1 minute. Mark it as Zero tube. (2) Add 1 ml of sample diluent to the freeze-dried standard tube, tighten the tube cap, let it stand at room temperature for 2 minutes, and gently mix it by inverting it upside down several times (or add 1 ml of sample diluent, let it stand for 1-2 minutes, and then mix it with a low-speed vortex for 3-5 seconds). Centrifuge at 1,000×g for 1 minute and collect the liquid at the bottom of the tube. (3) Gradient dilution: Take another 7 EP tubes and mark them as 1/2, 1/4, 1/8, 1/16, 1/32, 1/64 and blank respectively. First, add 0.3 ml of sample diluent to each EP tube. Then, add 0.3 ml of the standard solution from the Zero tube to the 1/2 tube and mix thoroughly. Then, add 0.3 ml of the standard solution from the 1/2 tube to the 1/4 tube and mix thoroughly. Then, add 0.3 ml of the standard solution from the 1/4 tube to the 1/8 tube and mix thoroughly, and so on. Note that the Blank EP tube contains only sample diluent. At this point, the concentrations of the standards in the eight EP tubes, from the Zero tube to the Blank tube, are 1000 pg/ml, 500 pg/ml, 250 pg/ml, 125 pg/ml, 62.5 pg/ml, 31.25 pg/ml, 15.625 pg/ml, and 0 pg/ml, respectively. 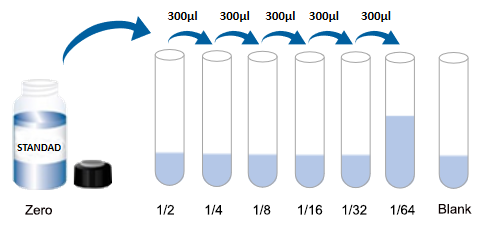 Note: Please store the dissolved standard in tube 0 at 2-8°C and use within 12 hours. Please use the diluted working solutions of other gradient standards within 2 hours. 3. Biotin-antibody working solution: Prepare within 30 minutes before the experiment. Prepare immediately before use. Not suitable for long-term storage. (1) Calculate the total volume of working solution required: 100ul/well × number of wells. (It is best to prepare 100ul-200ul more than the total volume.) (2) Centrifuge at 1000×g for 1 minute to collect the concentrated biotin-antibody at the bottom of the tube. (3) Dilute the concentrated biotin-antibody with antibody diluent at a ratio of 1/100 and mix thoroughly. (For example, add 10ul of concentrated biotin-antibody to 990ul of antibody diluent) 4. HRP-streptavidin (SABC) working solution: Prepare within 30 minutes before the experiment, prepare immediately before use, and not suitable for long-term storage. (1) Calculate the total volume of working solution required: 100ul/well × number of wells. (It is best to prepare 100ul-200ul more than the total volume.) (2) Centrifuge at 1000×g for 1 minute to collect the concentrated SABC at the bottom of the tube. (3) Dilute the concentrated SABC with SABC diluent at a ratio of 1/100 and mix thoroughly. (For example, add 10µl of concentrated SABC to 990µl of SABC diluent) Procedure Summary  Step 1: Add 100µl of standard or sample to the well, apply the film, and incubate at 37°C for 90 minutes. Step 1: Add 100µl of standard or sample to the well, apply the film, and incubate at 37°C for 90 minutes.Wash: Wash the plate twice. Do not soak. Step 2: Add 100µl of biotin-antibody working solution, apply the cover film, and incubate at 37°C for 60 minutes. Wash: Wash the plate three times, soaking for 1 minute each time. Step 3: Add 100µl of HRP-streptavidin (SABC) working solution, apply the cover film, and incubate at 37°C for 30 minutes. Wash: Wash the plate five times, soaking for 1 minute each time. Step 4: Add 90µl of TMB chromogenic substrate. Apply the cover film, and incubate at 37°C for 10-20 minutes (please use the TMB color development precision control method). Step 5: Add 50µl of reaction stop solution. Immediately read the OD450 value at 450nm and calculate. Detailed Procedure: When diluting samples and reagents, thoroughly mix them. It is recommended to create a standard curve for each test. 1. Assign standard, sample, and blank wells and record their positions. To minimize experimental error, it is recommended to assign standards and samples to duplicate wells. 2. Sample Addition: Add 100µl of each gradient standard to the standard wells, 100µl of appropriately diluted test sample to the sample wells, and 100µl of sample diluent to the blank wells. Apply the film and incubate at 37°C for 90 minutes. (Add the solution to the bottom of the microplate and gently shake to mix, avoiding contact with the tube walls and causing bubbles.) 3. Wash the plate twice: Remove the cover film, aspirate or shake off the liquid in the microplate, and tap the plate 2-3 times on clean absorbent paper. Add 350 μL of wash buffer to each well. Without soaking, discard the liquid in the well and tap the plate 2-3 times on absorbent paper. Repeat this wash step twice. 4. Add biotin-antibody working solution: Add 100 μL of biotin-antibody working solution to each well. Apply the cover film and incubate at 37°C for 60 minutes. 5. Wash the plate 3 times: Remove the cover film, aspirate or shake off the liquid in the ELISA plate, and tap the plate 2-3 times on clean absorbent paper. Add 350µl of wash buffer to each well, soak for 1 minute, discard the liquid in the well, and tap the plate 2-3 times on absorbent paper. Repeat the wash step 3 times. 6. Add HRP-Streptavidin (SABC): Add 100µl of SABC working solution to each well. Apply the cover film and incubate at 37°C for 30 minutes. At the same time, place the entire bottle of TMB in a 37°C incubator to equilibrate. 7. Wash the plate 5 times: Remove the cover film and wash the plate 5 times with wash buffer, following the procedure in step 5. 8. Add TMB chromogenic substrate: Add 90µl of TMB chromogenic substrate to each well, apply the cover film, and incubate at 37°C in the dark for 10-20 minutes. Turn on the microplate reader and preheat for 15 minutes. (Note: Do not use the sample reservoir used to prepare HRP conjugates. The reaction time can be shortened or extended depending on the actual color development, but should not exceed 30 minutes. Terminate the reaction when a good blue gradient appears in the standard wells. The color intensity should not be too weak or too strong, requiring precise control. 9. Adding Stop Solution: After color development, do not discard the liquid in the wells. Add 50µl of Stop Solution to each well. The color will immediately change from blue to yellow. The order of adding Stop Solution is the same as that of adding TMB substrate. 10. OD Measurement: Immediately read the OD450 value at 450nm using a microplate reader. (If your microplate reader has a selectable calibration wavelength, set it to 570nm or 630nm. The calibration reading is the OD450 value minus the OD570 or OD630 value. This method corrects for and removes the OD values of non-chromogenic substances.) Calculate the OD450 value for a more accurate result. If your microplate reader doesn't have a 570nm or 630nm wavelength, you can use the raw OD450 value. ) Calculation: 1. Take the average OD450 value of the replicate standard and sample wells (either the raw OD450 value or the calibrated reading) and subtract the OD450 value of the blank well to obtain the calculated value. 2. Use the four-parameter equation 4PL to plot a standard curve with concentration as the horizontal axis and OD450 value as the vertical axis (excluding the blank well values when plotting). You can also use the plotting software included with your microplate reader (such as SkanIt RE software for Thermo FC models) or Curve Expert 1.3 or 1.4 professional software to plot a standard curve. 3. Substitute the sample OD450 value into the standard curve to calculate the sample concentration. If the sample has been diluted, multiply by the dilution factor. Experimental Data and Standard Curve: This product has been tested by the Quality Control Department and meets the performance requirements in the user manual. (Laboratory humidity is 20%-60%, and temperature is 18°C-25°C. Before color development, equilibrate TMB to 37°C. After adding it to the ELISA plate wells, incubate at 37°C in the dark for 15 minutes.) Due to differences in specific experimental environments and operations, the following experimental data and standard curve are for reference only. Experimenters should establish a standard curve based on their own experiments. img style="display: block; margin-left: auto; margin-right: auto;" src="https://absin.oss-cn-shanghai.aliyuncs.com/absin-china/20240820/371cb0d5df9845309e2fbae17c9f1390.png" alt="" width="746" height="306" /> 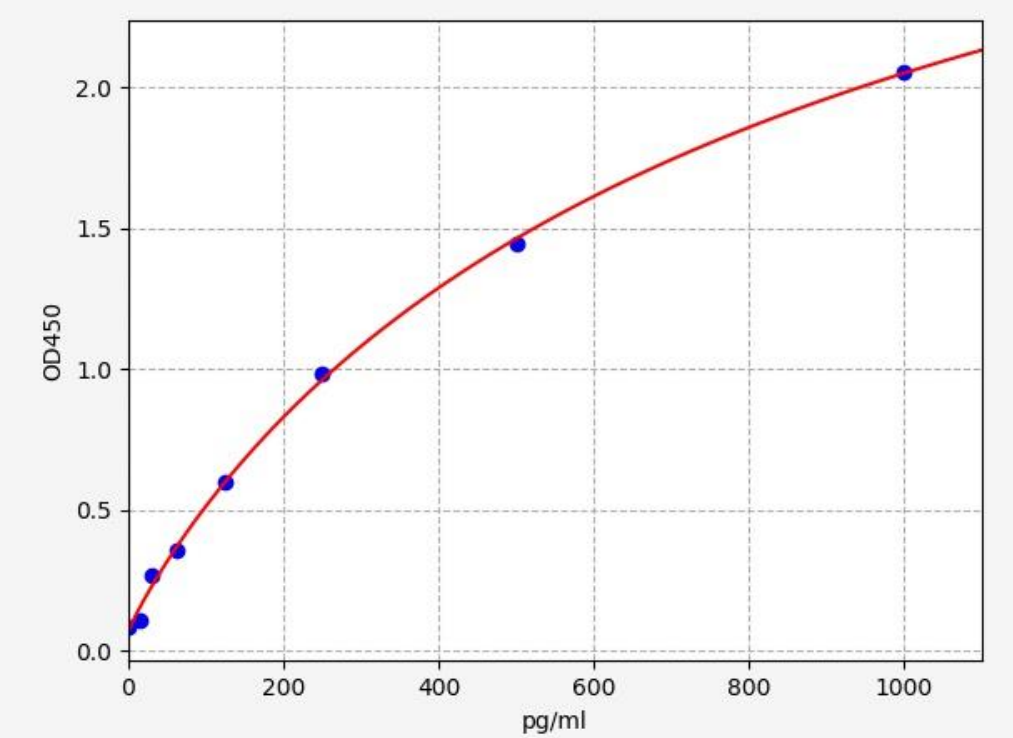 Precision: Recovery Linearity |
|||||||||||||||||||||||||||||||||||||
| Species Reactivity | Mouse | |||||||||||||||||||||||||||||||||||||
| Theory | This kit uses a double-antibody sandwich ELISA method, with a 4-hour assay duration. The ELISA plate included in the kit is pre-coated with an anti-IL-18 antibody. A standard and a moderately diluted sample to be tested are added to the corresponding wells, incubated, and unbound components are washed away. A biotin-detection antibody is added, which binds to the IL-18 bound to the coated antibody. After washing away unbound components, HRP-streptavidin (SABC) is added. Unbound components are further washed away, and TMB chromogenic substrate is added. TMB develops a blue color when catalyzed by horseradish peroxidase (HRP), which turns yellow upon addition of the reaction stop solution. The OD value is measured at 450 nm using a microplate reader. The IL-18 concentration in the sample is calculated by plotting a standard curve. The concentration of the target substance is directly proportional to the OD450 value. | |||||||||||||||||||||||||||||||||||||
| Synonym | Mouse Interleukin 18 (IL-18/IL18) ELISA Kit | |||||||||||||||||||||||||||||||||||||
| Description | Sensitivity: 9.375 pg/ml Detection wavelength: OD450 Specificity: Specific binding to IL-18, no significant cross-reaction with other analogs Detection method: Sandwich ELISA, Double Antibody Detection time: 4 hours (excluding equilibration and sample preparation time) |
|||||||||||||||||||||||||||||||||||||
| Composition |
|
|||||||||||||||||||||||||||||||||||||
| General Notes | 1. When using different kits, ensure they are labeled to prevent mixing components and potentially causing experimental failure. 2. After opening the kit, please refer to the component storage conditions table for the ELISA plate and standards (activity will decrease if exposed to moisture). 3. Use sterile, disposable pipette tips to aspirate reagents. After use, tighten the reagent bottle caps tightly to prevent microbial contamination and evaporation. 4. When manually washing the plate, avoid contact between the pipette tip or pipette with the plate wells. Inadequate washing or contamination can easily result in false positives and high background. 5. During the assay, prepare the reagents needed for the next step in advance. Add the reagents to the wells promptly after washing to prevent drying out and causing assay failure. 6. Do not use reagents from other batches or other sources with this kit without prior verification. 7. Do not reuse disposable pipette tips to avoid cross-contamination. 8. After sample addition, apply a film to prevent sample evaporation during incubation. Complete the incubation at the recommended temperature. 9. Please wear lab coats, masks, gloves, etc. during the experiment and take protective measures. Especially when testing blood or other body fluid samples, please follow the national biological laboratory safety protection regulations. | |||||||||||||||||||||||||||||||||||||
| Instructions | Used for in vitro quantitative detection of IL-18 in serum, plasma, cell culture supernatant or other biological samples. | |||||||||||||||||||||||||||||||||||||
| Storage Temp. | 2-8°C (unopened), do not freeze, valid for 12 months. | |||||||||||||||||||||||||||||||||||||
| Test Range | 15.625-1000pg/ml |