Product Details
Product Details
Product Specification
| Usage | I. Sample Collection, Preparation, and Storage 1. Serum: After placing whole blood samples at room temperature for 2 hours or at 4°C overnight, centrifuge at 1000×g for 20 minutes. Remove the supernatant for testing. Blood collection tubes should be disposable, pyrogen-free, and endotoxin-free. Store at -20°C or -80°C and avoid repeated freezing and thawing. 2. Plasma: Within 30 minutes of collection, centrifuge at 1000×g for 15 minutes at 2-8°C. Remove the supernatant for testing. EDTA-Na2 is recommended as the anticoagulant to avoid hemolysis or hyperlipidemia. Store at -20°C or -80°C and avoid repeated freezing and thawing. 3. Tissue Homogenization: Take an appropriate amount of tissue and wash it in pre-chilled PBS (0.01M, pH 7.0-7.2) to remove blood (lysed red blood cells in the homogenate will affect the measurement results). After weighing, mince the tissue and mix it with the appropriate volume of PBS (generally a 1:9 weight-to-volume ratio; the specific volume can be adjusted according to experimental needs and recorded. It is recommended to add protease inhibitors to the PBS). Pour the mixture into a glass homogenizer and grind thoroughly on ice. To further lyse tissue cells, the homogenate can be sonicated or freeze-thawed repeatedly (keep the sonication in an ice bath and repeat the freeze-thaw cycle twice). Finally, centrifuge the homogenate at 5000×g for 5-10 minutes. Remove the supernatant for analysis. 4. Cell Culture Supernatant: Centrifuge the cell supernatant at 1000×g for 20 minutes to remove impurities and cell debris. Remove the supernatant for testing and store at -20°C or -80°C, but avoid repeated freezing and thawing. 5. Urine: Collect the first morning urine (midstream) or 24-hour urine collection. Centrifuge at 2000×g for 15 minutes, collect the supernatant, and store the sample at -20°C. Avoid repeated freezing and thawing. 6. Saliva: Collect the sample using a saliva sample collection tube, then centrifuge at 1000×g for 15 minutes at 2-8°C. Remove the supernatant for testing, or aliquot and store at -20°C. Avoid repeated freezing and thawing. 7. Other biological samples: Centrifuge at 1000×g for 20 minutes, collect the supernatant, and store at -20°C. Avoid repeated freezing and thawing. Notes:1. Samples should be clear and transparent, and suspended matter should be removed by centrifugation. Hemolysis of the sample will affect the results, so hemolyzed samples should not be used. 2. Samples can be stored at 4°C if tested within one week of collection. If testing cannot be performed promptly, aliquot the sample into single-use portions and freeze at -20°C (for testing within one month) or -80°C (for testing within three to six months). Avoid repeated freeze-thaw cycles. Bring the sample to room temperature before experimenting. 3. If the concentration of the test substance in your sample is higher than the highest value of the standard, please dilute it appropriately based on the actual situation (it is recommended to conduct a pilot experiment to determine the dilution factor). 2. Pre-Assay Preparation 1. Remove the test kit from the refrigerator 30 minutes in advance and equilibrate to room temperature. 2. Dilute 25 μg of concentrated wash buffer to 1 μg of working solution with double-distilled water. Return any unused portion to 4°C. 3. Standards: Add 1.0 mL of Universal Diluent for Standards & Samples to the lyophilized standard. Tighten the cap and let stand for 10 minutes to fully dissolve. Gently mix to achieve a concentration of 2000 pg/mL. Perform serial dilutions to 2000 pg/mL, 1000 pg/mL, 500 pg/mL, 250 pg/mL, 125 pg/mL, 62.5 pg/mL, and 31.25 pg/mL. Use the standard diluent (0 pg/mL) as a blank well. Prepare the required amount of standard solution and set aside. It is recommended to add the prepared standard solution to the sample within 15 minutes; do not allow it to sit for extended periods. 4. Biotinylated Antibody Working Solution: Calculate the required volume for each experiment (100 μL/well; add 100-200 μL more). 15 minutes before use, dilute the concentrated biotinylated antibody (1:100) with Biotinylated Antibody Diluent to the working concentration. Use the same day. The dilution principle is to add 1 μL of concentrated biotinylated antibody to 99 μL of biotinylated antibody diluent and mix thoroughly with a pipette. 5. Enzyme Conjugate Working Solution: Before the experiment, calculate the required volume for the experiment (based on 100 μL/well; add 100-200 μL more when preparing). 15 minutes before use, dilute the concentrated HRP enzyme conjugate (1:100) with enzyme conjugate diluent to the working concentration for use that day. The dilution principle is to add 1 μL of concentrated enzyme conjugate to 99 μL of enzyme conjugate diluent and mix thoroughly with a pipette. 6. TMB Substrate - Use a pipette to aspirate the required volume of solution. Do not pour any remaining solution back into the reagent bottle. Note: 1. Before using the kit, ensure that all components are dissolved and mixed thoroughly. Discard any unused standard after reconstitution. 2. Concentrated biotinylated antibody and enzyme conjugates are small in size and may disperse throughout the tube during transportation. Before use, centrifuge at 1000 × g for 1 minute to allow any liquid on the tube walls or cap to settle to the bottom. Mix the solution by carefully pipetting 4-5 times before use. Prepare the standard, biotinylated antibody working solution, and enzyme conjugate working solution according to the required volume and use the corresponding diluents. Do not mix them. 3. Concentrated wash buffer may crystallize after removal from the refrigerator. This is normal. Dissolve the crystals completely in a water bath or incubator before preparing the wash buffer (do not heat above 40°C). The wash buffer should be at room temperature when used. 4. Samples should be added quickly, preferably within 10 minutes each time. To ensure experimental accuracy, it is recommended to use replicates. When pipetting reagents, maintain a consistent order of addition from one well to another. This will ensure the same incubation time for all wells. 5. During the wash process, any wash solution remaining in the reaction wells should be patted dry on absorbent paper. Do not place filter paper directly into the reaction wells to absorb water. Before reading, be sure to remove any residual liquid and fingerprints at the bottom to avoid affecting the microplate reader reading. 6. The color developer TMB should be protected from direct exposure to strong light during storage and use. After adding the substrate, pay attention to the color change in the reaction well. If a gradient is already obvious, stop the reaction early to avoid excessive color that affects the microplate reader reading. 7. The test tubes and reagents used in the experiment are disposable. Reuse is strictly prohibited, otherwise it will affect the experimental results. 8. Please wear a lab coat and latex gloves for proper protection during the experiment, especially when testing blood or other body fluid samples. Please follow the National Biological Laboratory Safety Protection Regulations. 9. Components of the kit from different batches cannot be mixed (except for wash buffer and reaction stop solution). 10. The enzyme label strips in the kit are detachable. Please use them in batches according to experimental needs. III.Operation Procedure 1. Before beginning the experiment, all reagents should be equilibrated to room temperature and all reagents should be prepared in advance. When diluting reagents or samples, mix thoroughly and try to avoid foaming during mixing. If the sample concentration is too high, dilute it with sample diluent to bring the sample within the detection range of the kit. 2. Add 100 μL of the standard or sample to be tested (if the sample needs to be diluted, refer to the sample dilution guidelines for dilution methods). Be careful not to create bubbles. Add the sample to the bottom of the ELISA plate well, avoiding contact with the sides. Gently shake to mix. Cover the plate or seal with film and incubate at 37°C for 80 minutes. To ensure the validity of the experimental results, use a fresh standard solution for each experiment. 3. Discard the liquid in the wells, spin dry, and wash the plate three times. Wash each well with 200 μL of wash buffer, soaking for 1-2 minutes. Spin off the liquid in the plate (or wash with a microplate washer). After the final wash, pat the plate dry on absorbent paper. 4. Add 100 μL of biotin antibody working solution to each well (can be prepared 15 minutes in advance). Cover the plate with film and incubate at 37°C for 50 minutes. 5. Discard the liquid from the wells and wash the plate three times. Wash each well with 200 μL of wash buffer, soak for 1-2 minutes, and discard the liquid from the plate (or use a plate washer). After the final wash, pat the plate dry on absorbent paper. 6. Add 100 μL of enzyme conjugate working solution to each well (can be prepared 15 minutes in advance) and incubate at 37°C for 50 minutes. 7. Discard the liquid from the wells and wash the plate five times. Wash each well with 200 μL of wash buffer, soak for 1-2 minutes, and discard the liquid from the plate (or use a plate washer). After the final wash, pat the plate dry on absorbent paper. 8. Add 90 μL of TMB chromogenic substrate solution to each well and incubate at 37°C in the dark for 20 minutes (shorten or extend the time depending on the actual color development, but do not exceed 30 minutes). 9. Add 50 μL of stop solution to each well to terminate the reaction (the blue color will immediately turn yellow). The stop solution should be added in the same order as the developer. To ensure accurate results, add the stop solution as soon as possible after the substrate reaction time expires. 10. Immediately measure the optical density (OD) of each well at 450 nm using a microplate reader. Preheat the instrument and set the assay program before use. Calculation of Results 1. Subtract the OD value of the blank well from the OD value of each standard and sample. If replicate wells are used, the average value should be used for calculation. 2. For ease of calculation, although concentration is the independent variable and OD value is the dependent variable, we still use the OD value of the standard as the horizontal axis (X-axis) and the concentration of the standard as the vertical axis (Y-axis) when plotting. At the same time, for the intuitiveness of the experimental results, the figure provides raw data rather than logarithmic values. Due to different experimental operating conditions (such as operators, pipetting techniques, plate washing techniques, and temperature conditions), the OD values of the standard curve will vary. The standard curve provided is for reference only. Experimenters need to establish a standard curve based on their own experiments. The OD value of the used sample can be used to calculate the sample concentration on the standard curve, and then multiplied by the dilution factor to obtain the actual concentration of the sample. It is recommended to use professional curve drawing software, such as curve expert.
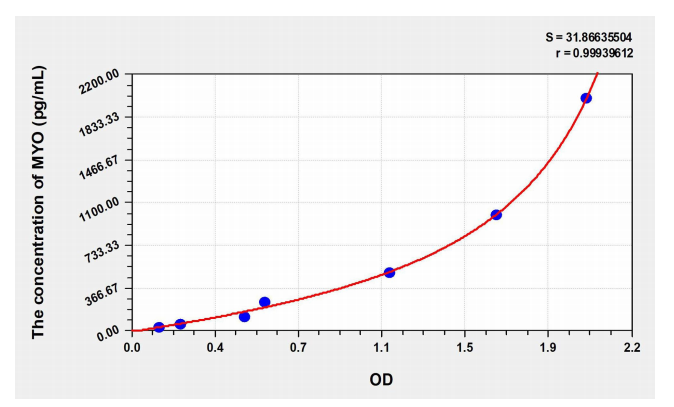 Note: This figure is for reference only Precision Intra-plate precision (precision within the assay): CV%<8% Three samples of known concentration were tested 20 times on each ELISA plate to evaluate intra-plate precision. Inter-assay precision (CV% <10%): Three samples of known concentrations were tested 40 times on three different ELISA plates to assess inter-assay precision. Recovery was performed by spiking different samples with known concentrations of mouse MYO to determine the recovery range and average recovery.
Linearity The samples spiked with mouse MYO were diluted 2-fold, 4-fold, 8-fold, and 16-fold, respectively. The recovery experiment was performed twice, and the recovery rate range was obtained.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species Reactivity | Mouse | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Theory | This kit utilizes a sandwich assay. A specific anti-mouse MYO antibody is coated in a 96-well microplate. A mouse MYO standard or sample is added to the wells, allowing the mouse MYO protein in the standard or sample to bind to the anti-mouse MYO antibody immobilized on the microplate. Biotinylated anti-mouse MYO antibody is then added. Unbound biotinylated antibody is washed away, and HRP-conjugated streptavidin is added. After thorough washing again, TMB substrate is added for color development. TMB converts to blue under peroxidase catalysis and to the final yellow under the action of acid. The intensity of the color is positively correlated with the amount of mouse MYO protein in the sample. The absorbance (OD) is measured at 450 nm using a microplate reader, and the sample concentration is calculated by plotting a standard curve. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonym | PVALB; MB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detection Type | Detects recombinant or native mouse MYO and does not cross-react with other related proteins | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Composition |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Notes |
1. If the entire kit is stored at -20°C, please place the kit at 4°C the night before the experiment. 2. Salt precipitation may occur when the concentrated wash solution is stored at low temperatures. When diluting, warm it in a water bath to help dissolve it. 3. A small amount of water-like substance may be present in the wells of a newly opened ELISA plate. This is normal and will not affect the experimental results. 4. This kit is for laboratory research and development use only and is not intended for use on humans or animals. 5. Reagents should be treated as hazardous substances and should be handled with care and disposed of properly. 6. Always wear gloves, lab coats, and protective glasses to avoid contact between skin and eyes with the stop solution and TMB. If contact occurs, rinse thoroughly with water. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Temp. | If the unopened kit is stored at 4°C, the shelf life is 6 months. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Test Range | 31.25-2000 pg/mL; Sensitivity: 13.2 pg/mL |